Dr. László Tabár, a radiologist and oncologist from Falun, Sweden, is known worldwide as the "father" of mammography screening. He is certainly one of the key people behind the current national screening program for breast cancer in Sweden, but his research has also formed the basis for the introduction of screening programs in many other countries.
In this interview, Tabár shares the fascinating story of why he has dedicated the past 50 years of his life to introducing and improving mammography screening, and thereby reducing mortality in breast cancer -- a journey colored by groundbreaking research results and success but also by opposition and a struggle to convince authorities of the benefits.
He gives us the story behind the introduction of mammography screening in Sweden and the intriguing results shown in two of his latest studies, published in 2018 and 2020, proving the benefits of screening independent of treatment regime -- something he calls "one of the greatest accomplishments in clinical cancer research during the past 50 years."
 Dr. László Tabár in action by his breast imaging PACS workstation. Images courtesy of Dr. László Tabár.
Dr. László Tabár in action by his breast imaging PACS workstation. Images courtesy of Dr. László Tabár.A key person in the history of mammography screening was Prof. Barbro Westerholm, chief of the National Board of Health and Welfare, who played a very important role in recommending nationwide screening after our group published its seminal trial in the Lancet. She is still a member of the Swedish Parliament and fighting for mammography screening -- nowadays for older women.
Key findings in the latest research from Dr. László Tabár
In the most recent study,1 published in March 2020, Tabár and his research team -- which consisted of a wide range of people from different mammography and radiology departments and cancer institutes, as well as biostatistical researchers -- could show that women who participated in mammography screening had a statistically significant 41% reduction in risk of death from breast cancer within 10 years of diagnosis and a 25% reduction in the rate of advanced breast cancers.
In total, 549,091 women ages 40 to 69 -- covering approximately 30% of the Swedish screening-eligible population -- were included in the study, making it the world's largest service screening study investigating the impact of participation in mammography screening on breast cancer death compared with women who did not participate.
Häger: Can you please walk us through how the screening program in Sweden has developed and what role your research has played?
Tabár: I have lived through the entire journey of Swedish mammography screening. In the mid-1970s, the low-dose mammography technique was introduced. Dr. Bengt Lundgren tested the method successfully in Gävleborg County, Sweden. This opened up for early detection of breast cancer and provided the possibility of treating the disease in its early, nonpalpable phase, i.e., before it became aggressive and spread to other organs.
When the method was proved to be suitable for mass screening, then we needed to answer the question of whether early detection and treatment in early phase of breast cancer will significantly decrease death from the disease. That required a randomized, controlled, population-based trial.
The largest randomized controlled study, the Swedish two-county trial (W-E trial)2 commenced in 1977 in Kopparberg (currently Dalarna) and östergötland. We tested the impact of "invitation to one-view mammography screening" in these two counties combined. A total of 134,867 women ages 40 to 74 were included in the trial, of whom 78,085 were invited and 56,782 were not invited. The latter of these comprised the control group.
The outcome was evaluated eight years later, and the result was astonishing. The invitation for screening reduced the death rate by a significant 31%.2 (The outcome of the screening itself could not be measured, since 15% of the invited women declined. Theoretically, if the women who declined invites were to be included, the reduction in the death rate would be 41%.) Additional randomized trials were carried out in Malmö, Gothenburg, and Stockholm, supporting the findings of the W-E trial.
The randomized study was conducted between 1977 and 1985, resulting in the National Board of Health and Welfare of Sweden recommending mammography service screening for all women ages 40 to 74 in 1986. The national mammography service screening program then commenced that year. The tipping point that led to the decision was when women themselves started to demand the screening service. The year was 1997 when the last county in Sweden started to offer screening, meaning that there was now nationwide coverage.
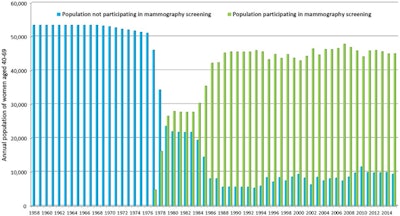 Figure 1. This graph, from one of Tabár's latest articles,3 published in 2018, illustrates the mammography screening participation rates over time (data from Dalarna County Council, from 1958 to 2015) for women ages 40 to 69.
Figure 1. This graph, from one of Tabár's latest articles,3 published in 2018, illustrates the mammography screening participation rates over time (data from Dalarna County Council, from 1958 to 2015) for women ages 40 to 69.You have dedicated your life to research on early detection of breast cancer through mammography screening. Why are these studies so important?
Breast cancer is a terrible disease and it kills too many women, but by carrying out these very demanding and complex studies, it has been proved that early detection combined with efficient treatment can significantly reduce mortality from the disease.
First, these studies are crucial to provide physicians with reassurance that participating in regular, high-quality mammography screening is the best way to reduce the risk of premature death from breast cancer. Second, women deserve an answer to the question of how participation in regular mammography screening will significantly improve their chances of surviving breast cancer.
There are many other benefits as well, such as less radical treatment, improved life quality, reduced cost for society, etc. A woman whose breast cancer has been detected at screening gains on average 16.5 years of life.
The studies proving the benefits of screening have also faced some resistance. Can you elaborate?
Yes, there are those who refuse to believe in the positive impact of early detection. Over the years, these people have endeavored to impede screening through different campaigns. But as evidence of the benefits of screening has accumulated over time, this group of people has diminished or become less active. In the end, all that matters are scientific data, and we have always backed our claims with facts.
Women are convinced that treating a 10-mm carcinoma must give better results and improved outcome than treating a 4-cm to 5-cm tumor. That explains the high participation rate when the county councils send out invites to regular screening.
Apparently, evidence is key. What have been the crucial factors that have enabled the research to be conducted?
Access to reliable data through the Swedish cancer and death registries has been the most important factor to study the number of women diagnosed with breast cancer and the cause of deaths. In our statistical analysis, these data provided the numerator. Sectra had a very important role back in the 1990s, when it signed agreements with the county councils concerning the collection and aggregation of data relating to the participation and nonparticipation of women in screening in connection with each invitation. These data provided the denominator in our research.
Let's dig into the two latest studies by your research group. First, the publication in 2018 received a lot of media attention. Can you elaborate on what was so remarkable?
Yes, indeed, the article3 published in 2018 was actually the most cited article in 2019 in the journal Cancer. What stood out from previous research was that we did not mix the women participating in screening with those who did not participate. We kept these groups separate when examining mortality rates. By doing so, we could show the benefits of the treatment and screening separately for these two groups. That made it unique.
Moreover, we used a new statistical method when calculating the risk of death from breast cancer in the 10 years following diagnosis, which put the date of diagnosis into focus, instead of using the conventional mortality calculation that focuses on the date of death. This way, we avoided contamination of data from breast cancers diagnosed in the past.
We showed that risk of death 10 years after diagnosis among women participating in screening was 60% lower compared with women who did not attend screening, even if they were invited. The breast cancer mortality rate was 47% lower within 20 years of diagnosis in women who underwent screening compared with those who did not. These results were above and beyond the impact of modern therapy -- a remarkable benefit of early detection.
The results demonstrate that women who have participated in mammography screening obtain significantly greater benefit from the therapy available at the time of diagnosis than those who have not participated.
Why did this study get such broad publicity?
The study challenged the position that mammography screening is not a key factor in reducing breast cancer mortality, that the chances of survival are mostly dependent on the treatment.
Before 2018, there was a common perception that mainly advances in adjuvant therapy and chemotherapy are responsible for improved breast cancer prognosis in screened populations, making screening programs less beneficial. But our results in 2018 demonstrated that the benefit of therapy is significantly greater for women who have participated in mammography screening. It is incorrect to attribute the mortality benefit to either early detection or treatment. The winner is the woman whose breast cancer is detected in screening and treated in an early phase.
The second article, published in March 2020, showed a similar result. Why was that study needed?
Reproducibility is basic in science. We needed to show that the results were valid across several counties. Therefore, in the 2020 article, we included 549,091 women in the age group 40 to 69 from nine counties, where the physicians and the personnel had been trained in Falun. This study covers approximately 30% of the Swedish screening-eligible population. (See figure 2.)
 Figure 2. Map of Sweden showing the locations of the nine counties involved in the study published in 2020.1
Figure 2. Map of Sweden showing the locations of the nine counties involved in the study published in 2020.1This study was also very well received globally. What conclusions did you make?
As in the previous 2018 study, we could confirm that early detection of breast cancer and treatment in an early phase results in a significant reduction in the breast cancer death rate in 30% of the country. The early detection in this case was a result of offering mammography screening to the population.
In numbers, we showed that women who participated in mammography screening had a statistically significant 41% reduction in risk of death due to breast cancer within 10 years and a 25% reduction in the rate of advanced breast cancers -- when contemporaneous comparison was made year after year between the two groups, following the same therapeutic guidelines. This made the study special since it could demonstrate the impact of early detection beyond the impact of therapy.
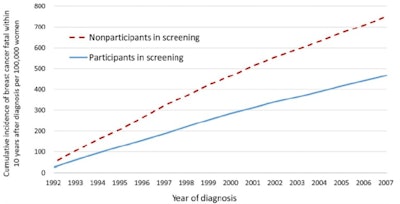 Figure 3. A graph from the most recently published study,1 illustrating the cumulative incidence of breast cancer that was fatal within 10 years of diagnosis for all nine counties combined.
Figure 3. A graph from the most recently published study,1 illustrating the cumulative incidence of breast cancer that was fatal within 10 years of diagnosis for all nine counties combined.The graph (see figure 3) speaks for itself, and the method we have used is bulletproof. We can now say that early detection is the key if we want to significantly reduce mortality in the population. This is a clear and easily understandable message for the medical community as well as for women.
You mentioned that the mortality reduction is independent of the treatment regime. Can you elaborate?
Because the comparison of participating and nonparticipating women was contemporaneous -- with mammography screening and breast cancer treatment taking place during the same period of time -- it is unaffected by potential changes in treatment of breast cancer over time. We can then conclude that breast cancer screening reduces the risk of death from the disease above and beyond current therapies in the absence of screening. One could otherwise claim the decreased mortality in breast cancer is due to better treatments alone and not improved by screening. Both are needed, and our study shows that detecting breast cancer early on is improving treatment outcomes.
A final question: Do you have any recommendations to those managing screening programs?
One could express in brief that both the "providers" (healthcare personnel) and the "consumers" (women) need regular information, training, and further education about the results of modern clinical cancer research. In addition, the introduction of new imaging methods, such as preoperative breast MRI for each breast cancer patient and automated breast ultrasound as an adjunctive method to full-field digital mammography, should be implemented for examining women with dense breast tissue, since we are missing every third invasive cancer hiding in the dense breast tissue. Large-section histopathology needs to replace the archaic, currently used small-section histopathology method.
I would also like to mention the results of our third article,4 published in May 2020 in the Journal of Medical Screening, in the U.K. We wrote this article because the relative risk values in the article from 20201 showed large variations in the different counties, giving the impression that the service to women varied significantly in the nine counties. The third article4 clarified this issue and concluded that the physicians and the personnel in all nine counties provided the very same benefit for those women who attended screening; the survival rates, however, were not only significantly poorer but also varied enormously among women who did not attend screening.
These results are important since they prove that women in 30% of the country receive the same high-quality mammography service, whether it be in Stockholm, central Sweden, or the north of the country. But they also show that breast cancer survival is significantly poorer among women who did not attend screening and that modern therapeutic regimens could not influence breast cancer the same way among women who did not attend screening compared with those who did. This variation in mortality between participating and nonparticipating women is illustrated by figures 4 and 5 below.4
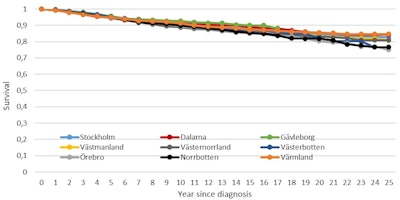 Figure 4. Survival rates of breast cancer patients participating in mammography screening, by county.4
Figure 4. Survival rates of breast cancer patients participating in mammography screening, by county.4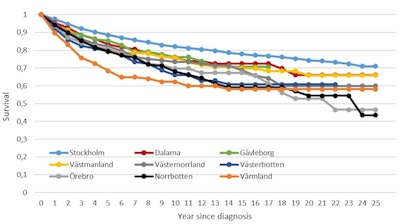 Figure 5. Survival rates of breast cancer patients not participating in mammography screening, by county.4
Figure 5. Survival rates of breast cancer patients not participating in mammography screening, by county.4References
1. Duffy SW, Tabár L, Yen AM, et al. Mammography screening reduces rates of advanced and fatal breast cancers: Results in 549,091 women. Cancer. 2020;126(13):2971-2979.
2. Tabár L, Fagerberg CJ, Gad A, et al. Reduction in mortality from breast cancer after mass screening with mammography. Randomized trial from the Breast Cancer Screening Working Group of the Swedish National Board of Health and Welfare. Lancet. 1985;1(8433):829-832.
3. Tabár L, Dean PB, Chen TH, et al. The incidence of fatal breast cancer measures the increased effectiveness of therapy in women participating in mammography screening [published online ahead of print, November 8, 2018]. Cancer. 2019;125(4):515-523.
4. Tabár L, Chen TH, Yen AM, et al. Early detection of breast cancer rectifies inequality of breast cancer outcomes [published online ahead of print, May 5, 2020]. J Med Screen. 2020.
Disclosure: Simon Häger is a market strategist for breast imaging and imaging IT developer Sectra. He is responsible for watching trends and customer needs within everyday imaging diagnostics. He has a background in medical imaging that spans digital pathology, radiology, and enterprise image management. This article was adapted from one originally published on Sectra's LinkedIn page by the author.
The comments and observations expressed herein do not necessarily reflect the opinions of AuntMinnie.com or AuntMinnieEurope.com, nor should they be construed as an endorsement or admonishment of any particular vendor, analyst, industry consultant, or consulting group.