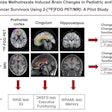
Patients with recurrent prostate cancer had better outcomes when PET with the Axumin radiopharmaceutical was part of the imaging package guiding their radiation therapy, according to a study presented on October 26 at the American Society for Radiation Oncology (ASTRO) annual meeting.
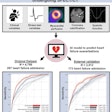
In the single-center, randomized study of prostate cancer patients after prostatectomy being considered for radiation therapy, those who were assessed with PET enhanced with the radiopharmaceutical fluciclovine F-18 (Axumin, Blue Earth Diagnostics) plus conventional imaging had significantly better post-treatment outcomes, which was defined as failure-free survival at three years (75.5%), compared with conventional imaging alone (63%).
Furthermore, longer-follow up showed that the failure-free survival rate was also significantly longer at four years for the arm that was assessed with standard imaging plus PET with Axumin (75.5% compared with 51.2%), Emory University researchers reported at the meeting, held October 24-28 in a virtual format.
The research shows that advanced molecular imaging such as with Axumin PET is a helpful add-on to standard imaging to guide decisions about which patients can benefit from radiation therapy following prostatectomy, Dr. Ashesh Jani, professor of radiation oncology at the Winship Cancer Institute of Emory University, and colleagues concluded.
Management of cancer patients after prostatectomy can be challenging because prostate-specific antigen (PSA) levels continue to rise in a large subset (20% to 40%), signifying a recurrence. But there is a high failure rate with postsurgery radiation therapy, Jani noted.
The study reported at ASTRO 2020 -- Emory Molecular Prostate Imaging for Radiotherapy Enhancement (EMPIRE-1) -- enrolled a total of 165 men with adenocarcinoma and detectable PSA and a negative bone scan, with no extra-pelvic metastases on CT or MR. In one arm, radiation therapy treatment was planned based on standard imaging, that is bone scans with CT or MR. In the other arm, treatment was guided based on the PET with Axumin scans, after standard imaging.
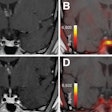
Molecular imaging helps identify patients who have extrapelvic disease and are unlikely to benefit from radiation therapy as well as pinpoint the location of a prostate cancer recurrence, the researchers noted. Furthermore, the researchers reported that the safety results were acceptable, with no significant differences in severe genitourinary or gastrointestinal adverse events between the two study arms.
"Though previous PET studies have led to changes in how prostate cancer is managed, an ensuing improvement in patient outcomes had not been demonstrated until this point," according to a statement from ASTRO about the data.
During a press briefing at the ASTRO meeting, moderator Dr. Sue Yom, PhD, described the difference of 12% for the primary endpoint of conventional imaging compared with conventional imaging plus PET as "very impressive," adding that it's very rare to see that kind of change for an imaging intervention on hard cancer endpoints in a trial.
The authors advised including PET in the assessment of patients with rising PSA levels to get the best results, but they also acknowledged that although fluciclovine was approved by the U.S. Food and Drug Administration in 2016 and has been incorporated into national cancer guidelines for cancer restaging, it is still not widely used for radiation treatment planning. Research shows that some insurers are reluctant to cover PET, including for use in the assessment of recurrent prostate cancer, citing a lack of supporting data.
"This study gives us data to make that case," said Yom, professor of radiation oncology and otolaryngology at the University of California, San Francisco.
Meanwhile, a new method that targets prostate-specific membrane antigen (PSMA) with a gallium-68 radiopharmaceutical in PET imaging has come to the fore. Emory researchers are testing the PSMA tracer head to head against fluciclovine PET in the EMPIRE-2 study, which is set to enroll 140 patients with a similar profile as in EMPIRE-1. Telix International submitted the PSMA tracer to the FDA for approval in a new drug application filed in September.