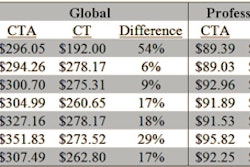
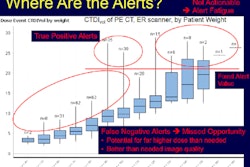
Several GE Healthcare CT scanners have been cleared by the U.S. Food and Drug Administration (FDA) to perform low-dose lung cancer screening.
All new GE CT scanners at 64 slices and higher, as well as virtually all the 16-slice CT scanners that GE sells, are qualified systems under the clearance and will include the screening option.
In addition, low-dose CT lung cancer screening is also available to thousands of other scanners currently in use, GE said. The screening option includes new low-dose screening reference protocols tailored to the CT system, such as patient size, and recommendations from a wide range of professional medical and governmental organizations.
GE is also noting that the new protocols can utilize GE's dose reduction technologies such as adaptive statistical iterative reconstruction (ASiR), ASiR-V, and Veo, which are designed to reduce image noise that can be problematic for physicians looking for small lung nodules.