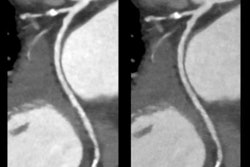
Canon Medical Systems has announced that the U.S. Food and Drug Administration (FDA) has cleared its Compressed Speeder technology for use with 3D sequences on Canon's Vantage Orian 1.5-tesla MRI system.
Compressed Speeder 3D can now be used to speed up MRI scan times for 3D sequences for use in scans for surgical planning or orthopedic applications. The solution uses undersampled data and interactive reconstruction to create full-resolution images. As a result, scans can occur up to 16 times faster, Canon noted.
Compressed Speeder 3D is available as an option for a new version of M-Power software for the Vantage Orian 1.5-tesla system. The technology can also be combined with Fast3D, a new technique to speed up exam time even further.