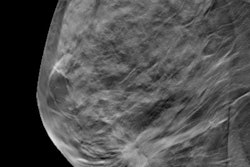
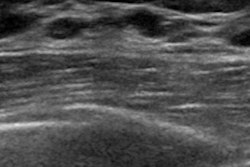
iCAD has received U.S. Food and Drug Administration (FDA) clearance for its ProFound AI artificial intelligence (AI) software for analyzing digital breast tomosynthesis (DBT) studies.
ProFound AI is designed to help radiologists analyze DBT exams, which can be a time-consuming task. The algorithm is trained to detect malignant soft-tissue densities and calcifications while providing radiologists with scoring information that indicates the likelihood that a detection or case is malignant. The algorithm was trained on a large dataset of clinical images, according to iCAD.
Research presented at RSNA 2018 indicated that radiologists using the software saw their cancer detection rate increase by 8%, with an average 50% reduction in reading time and a 7% drop in recall rate. What's more, radiologists with different levels of experience benefited from using the software, according to the company.
ProFound AI is available for leading DBT systems in the U.S., Canada, and Europe.