Radiostereometry technology provider Halifax Biomedical has received U.S. Food and Drug Administration (FDA) 510(k) clearance for its radiostereometry upgrade.
The upgrade -- the first product to be launched under a joint development agreement between Halifax and GE Healthcare -- enables GE's Discovery XR656/XR656 Plus x-ray imaging units to perform in vivo 3D measurements, according to Halifax.
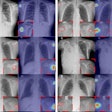
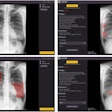
The system consists of an L-arm imaging device with an x-ray tube that synchronizes with the XR656 x-ray tube and GE imaging plates to take two digital x-rays at the same time, Halifax said. Designed to take up minimal floor space, the upgrade can be positioned to perform both standing and table exams, according to the firm.
Its image processing software uses calibration hardware integrated into the L-arm as a reference, yielding 3D precision in the range of 0.05 mm for measuring bone and implant positions within the body, Halifax said. The upgrade is now available for current XR656 customers in the U.S.