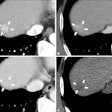
Bayer has secured 510(k) clearance from the U.S. Food and Drug Administration (FDA) for the use of single-dose vials of Visipaque (iodixanol) contrast agent with its Medrad Centargo CT Injection System.
The FDA has also cleared the use of single-dose vials of contrast agents that it previously approved for use in Imaging Bulk Package (IBP) with the Centargo, specifically Ultravist (iopromide), Isovue (iopamidol), Optiray (ioversol), and Omnipaque (iohexol).