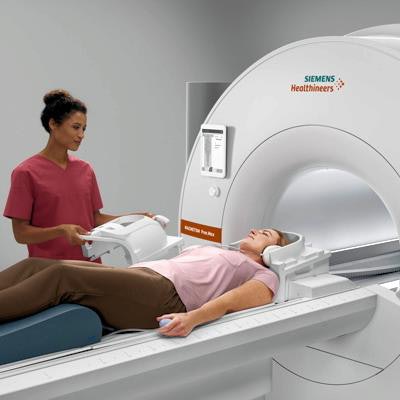
Siemens Healthineers has garnered U.S. Food and Drug Administration (FDA) clearance for Magnetom Free.Max, a 0.55-tesla, wide-bore MRI scanner that features artificial intelligence (AI) and new image-processing techniques.
First showcased at RSNA 2020, Free.Max has an 80-cm bore and is less than 80 inches high and weighs less than 3.5 tons, according to the vendor. It also uses less than 1 liter of helium and has no quench pipe, according to the vendor.
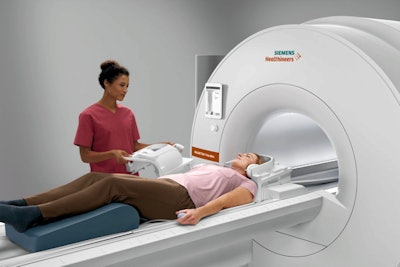 Siemens' Magnetom Free.Max 0.55-tesla MRI scanner. Image courtesy of Siemens Healthineers.
Siemens' Magnetom Free.Max 0.55-tesla MRI scanner. Image courtesy of Siemens Healthineers.The scanner also employs Deep Resolve, a set of algorithms that perform targeted denoising and utilize deep learning to provide sharper, higher-resolution images, Siemens said. In addition, the company said it has also included myExam Companion, a software package that incorporates AI to improve workflow and achieve consistent results.
The vendor said that the system's low weight and size could enable its use in orthopedic centers, emergency rooms, and intensive care units.


















