Technetium Labeled Cardiac Imaging
Tc-99m-Sestamibi (2-methoxyisobutyl-nitrate)
and Tc-99m-Tetrofosmin
Advantages
Technetium agents have the following advantages when compared to thallium:
1- Energy: The 140keV photon is optimal for gamma camera imaging and can produce higher quality images due to less attenuation, less scatter, and a brighter flash within the scintillation detector (Thallium uses low energy Mercury photons [60-83keV] which have high tissue attenuation).
2- Shorter half-life: Technetium has a 6 hour half-life, as opposed to 73 hours for Tl-201. Therefore, one can administer a 10 to 15 times higher dose than thallium which results in a higher count rate [47]. With the use of a larger dose, images can be obtained in a shorter period of time. High count rates also permit the use of gating to assess wall motion and ejection fraction [47].
3- Production: Technetium is available via a generator 24 hours a day. Thallium is cyclotron generated and requires off-site delivery.
Physiology & Pharmacology
When atherosclerotic narrowing occurs in a coronary artery, the reduction in perfusion pressure causes the coronary arterioles to dilate in order to maintain resting coronary blood flow [133]. Blood flow through a diseased coronary artery at rest is not decreased until the stenosis exceeds 85-90% of the luminal diameter [132,133]. At this point, the coronary arterioles are fully dilated [133]. Thus, resting regional myocardial blood flow is usually homogeneous, even in the presence of significant coronary artery stenosis [132]. Therefore a resting perfusion defect on SPECT imaging indicates a critical coronary artery stenosis, a myocardial scar from infarction [131]. Rest perfusion defects have also been shown to carry prognostic significance [136]. In one study, as the percent of myocardium with resting defects worsened, overall coronary event rates increased (a 3% increase in risk of CAD events was observed for every 1% of the myocardium with resting defects) [136].
During exercise coronary vessels that contain
smooth muscle in their walls will dilate (particularly the
coronary arterioles), the coronary vascular resistance will
decrease, and coronary blood flow will increase [133].
Coronary reserve is the ability to increase coronary blood
flow in response to metabolic demand. A greater than 80-85% stenosis is required to cause resting
ischemia [134], however, with maximal vasodilatation, this
threshold is decreased to 50% [197]. In other words, the
coronary reserve is decreased when a coronary artery stenosis exceeds 40-50% (i.e.: despite
maximal arteriolar dilatation, flow can no longer be increased
to meet metabolic needs) [132,133,134]. Therefore, compared to
rest imaging, an exercise exam is more likely to detect
regions of ischemia. Adequate exercise stress is essential for
the detection of ischemia. Adequate exercise during a
treadmill test is generally defined as the ability to perform
a workload of greater than or equal to 7 METS (completion of
the first two stages of the Bruce protocol or equivalent) and
acheive a heart rate greater than or equal to 85% of the
predicted maximal heart rate [178]. Inability to reach 85% of
the maximal predicted heart rate during exercise may decrease
the sensitivity of the exam [135]. Although there is similar
diagnostic accuracy for exercise or pharmacologic stress SPECT
imaging, an advantage of exercise is the additional prognostic
information gained from the stress test [178]. The most
important prognostic variables are exercise duration (METS
achieved) and the extent of exercise-induced ST-segment
depression [178].
Of course, when a coronary stenosis is present, the lumenal narrowing generated by the lesion is only partially responsible for the decreased coronary flow reserve [133]. Capillary derecruitment distal to the stenotic vessel occurs in an attempt to maintain a constant capillary hydrostatic pressure in the face of maximal arteriolar dilatation [133]. This derecruitment results in a decreased capillary surface area, which causes a reduction in the extraction of the radioisotope and the consequent perfusion defect seen on imaging [133]. The degree of capillary derecruitment distal to a stenosis during hyperemia is proportional to the severity of the stenosis [133].
To prevent medications from masking ischemia, beta-blockers should be withheld for 48 hours prior to the exam, and calcium channel blockers and long-acting nitrates should be held for 24 hours.
Tc-Sestamibi:
Sestamibi is a lipophilic monovalent cation (an isonitrile compound) [104]. It enters the cell via passive diffusion across plasma and mitochondrial membranes. It is postulated that MIBI accumulates within the mitochondria and cytoplasm of cells on the basis of electrical potentials generated across the membrane bilayers. At equilibrium it is sequestered largely within mitochondria (90%) by a large negative transmembrane potential. The agent is unmetabolized [11] and is fixed intracelluIarly as long as cell membrane integrity is intact and nutrient blood flow persists. It will not be extracted by non-viable myocardium. In plasma, less than 1% of the tracer is protein bound. Prior to use, the radiochemical purity of Tc-Sestamibi should be greater than 90%. Ethanol is the solvent used for quality controlusing paper chromatography. Sestamibi migrates to the top of the strip. Adverse reactions to Sestamibi are rare (less than 0.5%) and there have only been a couple of case reports of anaphylaxis [105].
The first pass extraction fraction for Tc-Sestamibi is approximately 65% (lower than that for thallium, which has an extraction fraction of about 85%) [47]. About 1-2% of the injected dose localizes to the myocardium at rest. The lower extraction fraction is overcome by the larger dose of MIBI which results in a higher count rate. Additionally, after initial extraction, blood levels fall rapidly which provides excellent target to background ratios as long as liver and bowel do not interfere with the image.
The uptake of Sestamibi in the myocardium is proportional to blood flow in the physiologic flow range. However, there is a plateau in extraction at higher flow rates, most likely because the tracer enters the cell via diffusion [22,47]. This plateau in extraction occurs at a lower level of increased flow above baseline compared to Tl-201 [47]. Because of the extraction plateau, Tc-sestamibi will underestimate blood flow at high flow rates (>2.0mL/min. per gram or about 2.5-3 times baseline flow [22,47]). Such high flow rates are encountered when using pharmacologic stress agents such as dipyridamole or adenosine. Therefore, it is theoretically possible for a mild to moderate stenosis which does not significantly impair coronary artery flow reserve to be overlooked with sestamibi vasodilator stress imaging [22]. Even so, the higher spatial resolution and lack of redistribution may offset the expected finding of slightly lower defect contrast associated with this reduced extraction at higher flow rates when compared to thallium. Overall, Tc-sestamibi is primarily a perfusion agent- it provides only limited information regarding myocardial viability and will frequently overestimate the amount of myocardial scarring.
Myocardial clearance of Tc-sestamibi is slow and the agent does not redistribute to a degree that can be imaged clinically [16]. Note (the following is certainly beyond what would ever be expected of a radiology resident): Heart House Course, Bethesda '93: Sestamibi undergoes minimal (about 20%) redistribution primarily within the first 20 to 60 minutes following injection. This may impact on lesion detection as the differential washout between the normal and ischemic myocardium may result in a reduction in defect size or severity with time. Therefore, their recommendation was to begin imaging 15 minutes following stress injection, and 60 minutes following rest injection. The tracer is retained in normal myocardium for several hours (myocardial clearance T1/2 is about 5 hours). Myocardial washout of MIBI is increased in patients with chronic heart failure [104] and in patients with hypertrophic cardiomyopathy- particularly those patients with impaired contractile reserve [159]. An area of reverse-redistribution can be seen following PTCA in patients with acute MI- indicating that the ability of myocytes to retain the tracer may be impaired in stunned myocardium (possibly related to loss of the normal membrane potential or mitochrondrial injury) [84].
The primary route of excretion is hepatobiliary (33%). Clearance via
this route is prompt (T1/2 of approximately 30 min.).
Unfortunately, scatter from both liver and bowel activity can
interference with visualization of the inferior ventricular
wall. This activity can be backprojected
over the inferior wall during image reconstruction. High
hepatic uptake can also create artifactual
perfusion defects in the inferior and inferoseptal
walls [1]. Finally, this activity may also severely disrupt
image normalization programs. There is about 25% renal
clearance of the agent. The critical organ is the upper large
intestine, which receives about 5.4 rads
per 30 mCi dose of Sestamibi.
Total body dose is about 500 mrem
to 1.75 rem
(8-17.5 mSv) for a 1 day
rest-stress exam [78,123].
Tc-MIBI does not concentrate in breast milk to any significant degree and cessation of breast feeding is not necessary following a Tc-MIBI exam. Close contact with the infant, however, should be avoided [17].
Technetium-99m-Tetrofosmin [1,2-bis [bis
(2-ethoxyethyl) phosphino]
ethane]
Tetrofosmin is a lipophilic, cationic, diphosphine which is rapidly cleared from the blood following intravenous administration [47]. The agent requires only a 15 minute incubation at room temperature for preparation. The agent is taken up by the heart, skeletal muscle, liver, spleen, and kidneys in proportion to blood flow and viability [24]. Between 1 to 1.5% of the injected dose localizes to the myocardium. The uptake mechanism is membrane-potential driven diffusion independent of cation channel transport [24]. The agent accumulates within mitochondria similar to Tc-sestamibi [47]. Myocardial uptake is decreased by metabolic inhibitors that cause severe cell injury or cell death [47].
The agent does not redistribute to any significant degree, but the higher lipophilicity may explain its higher initial uptake and faster washout [24]. The biological half-life for tetrofosmin in normal myocardium is 278 +/- 32 minutes, which is shorter than sestamibi (680 +/- 45 minutes) [24]. Hepatic uptake is lower than with Tc-Sestamibi and it also clears more quickly which can permit imaging sooner after injection (as soon as 5-15 minutes after exercise injection and 30 minutes for rest injection) [19,20,24,111].
The mean first pass extraction fraction is about 54% [47]. As with Tc-Sestamibi there is decreased tracer extraction compared to Thallium at high flow rates (such as that encountered with pharmacologic stress). The extraction of Tetrofosmin plateaus at a flow rate of approximately 2.0 mL/min/gm3 (or about 1.5 times normal flow [47]). The heart to lung contrast ratio is similar to sestamibi between 30 to 60 minutes post-injection [24]. Similar to Tc-Sestamibi, lesion conspicuity for mild to moderate stenoses may be less apparent than for Tl201 [47]. The typical total-body effective dose from a one day rest-stress exam using 10 mCi + 30 mCi is about 1.06 rem (10.6 mSv) [78]. Adjustments to the amount of radiotracer used can be made based upon patient size (i.e.: increase the doses to 15 mCi + 45 mCi for patients over 300 lbs) [85]. Patients over 350 pounds should undergo a two-day protocol [85].
Some authors advocate early (15 minute) post stress imaging with Tetrofosmin due higher diagnostic accuracy with better defect detection [153,163]. This may be related to a differential regional Tetrofosmin wash-out from non-ischemia (more rapid washout) versus ischemic regions that decreases perfusion defect contrast over time [153]. Also- earlier imaging may detect more patients with abnormal post stress LVEF [163].
Sensitivity&
Specificity
The sensitivity/specificity for SPECT Tc-Sestamibi
and Tc-Tetrofosmin in the detection
of coronary artery disease are
almost identical to thallium (sensitivity of about 90% and
specificity of about 80%) [2]. With the use of ECG gating,
improved imaging protocols and image quality, the diagnostic
accuracy of MPS for the detection of angiographically
significant CAD is high (sensitivity 87-89% and specificity
73-75%) [142].
A normal MPS exam, in a patient with intermediate to high
likelihood of CAD, predicts a very low rate of cardiac death or
non-fatal MI (= 1%/year) [142]. Even in patients with known CAD,
a normal MPS exam indicates a favorable prognosis (although the
rate of cardiac events is higher than for patients without known
CAD) [198, 199].
However, for patients with underlying cardiac risk factors
(hypertension, diabetes, and smoking) the all-cause mortality
can be higher and the risk is increased further with more than
onme risk factor - 0.6% with one risk factor, 1.3% with two risk
factors, and 1.7-1.8%/year compared to 0.2% when all three risk
factors are absent [164,198,216]. A
meta-analysis found that diabetic patients were at a higher risk
for cardiac events following a negative exam with an annualized
event rate of 1.6% [212]. Other authors have identified
additional synergistic risk factors that can affect the event
rate including LVEF < 45%, poor exercise capacity (excersize
duration of less than 6 minutes), obesity, higher resting HR,
LVH, abnormal ECG, and atrial fibrillation [208,216]. Also- for
patients with normal perfusioin scans, the relative risk for
total cardiac and all-cause death/MI events significantly
increases when the coronary artery calcium score is greater than
400 [201]. Additionally, a post-stress LVEF = 45% is also
associated with a higher cardiac event rate, even in the
presence of a normal scan (particularly in diabetic patients)
[205,216].
The Duke tredmill score (DTS) can provide prognostic risk
assessment in patients with suspected CAD [227]. The score is
calculated as follows: exercise duration (in minutes) - (5 x mm
ST segment deviation) - (4 x angina index [0 for no angina, 1
for angina, and 2 for exercise-limiting angina]) [227]. Patients
are categorized as low risk (< 0.5%/yr; score = +5),
intermediate risk (0.5-5%/yr; score +4 to -10), and high risk
(>5%/yr; score = -11) [227]. Guidelines suggest that patients
with high risk DTS be referred for coronary angiography [226].
However, younger, asymptomatic, and hypertensive patients with
LV hypertrophy (due to exaggerated magnitude of ST depression)
can have their risk over-estimated [226]. For patients with
high-risk Duke tredmill scores, up to 29% of patients will have
normal myocardial perfusion exams and these patients have been
shown to have a lower cardiovascular event rate compared to high
risk DTS patients with abnormal perfusion scans (4.4% vs 15% for
MI or cardiovascular mortality) [226].
Perfusion imaging of female patients poses certain challenges- specifically women have a relatively smaller heart size, there is attenuation associated with breast tissue, and they have a lower prevalence of CAD [88]. The reported overall accuracy for technetium agent perfusion imaging in women is sensitivity 71-83%, specificity 80-86%, and accuracy 82% (with higher sensitivity for multi-vessel compared to single vessel disease and for higher grade stenoses) [89]. However, a meta-analysis suggested that the diagnostic accuracy of SPECT MPI is similar for both men and women [195].
The use of attenuation correction (AC) can improve the
specificity, normalcy, and accuracy of the perfusion exam [53,54,68,75,101]. However, attenuation
correction (AC) can also create false-positive examinations with
AC induced apical thinning and truncation artifacts [126]. In
one study, there was no significant
diagnostic differences between automated itative exam
analysis processed with and without AC [126]. Radioisotope-based
attenuation correction requires sources that must be replaced as
they decay [101]. Depending on their age, a Gd-153 line source
used for attenuaiton correction typically exposes the patient to
an effective dose of about 0.001-0.01 mSv (less than that of a
single CXR) [178].
Attenuation correction can also be accomplished with hybrid
SPECT systems that utilize CT [69,75,101].
CT attenuation correction has been shown to improve the
diagnostic yield of SPECT imaging for detecting significant CAD
(greater than 50% stenosis) [75].
However, misalignment between the transmission and emission
studies can be a major source of artifacts [97] and misalignment
can be found in up to 42% of studies [98,103]. In another study,
misregistration of more than 1 pixel
was found in 73% of studies, and more than 2 pixels in 23% of
studies [147]. Misregistration can
occur in the cephalad/caudal,
dorsal/ventral, and left/right axes or in a combination of
planes [147]. Polar map scoring appears to be least affected by
cephalad/caudal shift and most
affected when the lateral or anterior myocardial walls on SPECT
overlapped lung tissue on the CT scan [147]. The most
significant effects on polar map scoring was associated with a 3
pixel ventral shift that resulted in decreased activity in the
lateral wall, and a caudal shift that lead to decreased uptake
in the anterior wall [147]. Despite this misregistration,
the exam results may not be significantly affected [103]
However, a careful review of image registration should be
performed to avoid reconstruction artifacts due to misregistration [103]. A slow CT
acquisition with a 4 second rotation during free breathing may
provide better image registration between the SPECT and CT data
sets [73], however, other authors note no significant difference
when using a high-speed CT [184]. Severe CT related artifacts
(from beam hardening, such as those related to metal implants)
can render up to 8% of studies uninterpretable
[103,184]. One added benefit of using CT for attenuation
correction is that potentially significant abnormal findings can
be found in up to 10.5-12% of patients on the non-diagnostic CT
portion of the exam [87,225]. However, the radiation exposure
using CT attenuation is higher with effective doses of 0.05-1mSv
depending on the scanner type and scan parameters employed
[178].
Same
day Rest and Stress study
An initial rest exam followed by the stress study was found to be more effective for determining the presence of reversible abnormalities [3]. A stress/rest sequence results in an increased number of ischemic segments incorrectly being identified as fixed defects (7% false-negatives) [4]. Additionally, in the stress-rest protocol, the rest portion of the exam may not be considered to be a "true" rest study as it follows a period of exercise and this may contribute to the overestimation of scar.
Patients should fast for 4 to 6 hours prior to the exam in
order to decrease heptic and GI
activity [85]. The first injection (rest study) is low dose
(8mCi), while the second or stress injection is high dose
(22mCi- approximately 3 times the rest dose in order to
overwhelm activity remaining in the myocardium) and follows the
first by 2-3 hours to permit time for some decay of the agent.
A sliding dose scale based upon patient weight is used at many
centers with guidelines recommending administration of an
additional 0.31 mCi/kg Tc-99m agent in patients weighing over 70
kg [178]. For a one day rest/stress study the typical doses are
8-12 mCi for the rest exam and 24-36 mCi (> 3x's resting
dose) for the stress study [178]. Another recommendation is: for
patients with BMI <= 25 kg/m2 - 8 mCi rest and 24
mCi stress; for BMI >25-30 kg/m2 - 9 mCi rest and
27 mCi stress; for BMI > 30-35 kg/m2 - 10 mCi rest
and 30 mCi stress; and for BMI > 35 kg/m2 - 12 mCi
rest and 36 mCi stress [223].
Non-lubricated syringes should be used to administer the agent
due to variable adherence of the agent to silicone lubricant
which can affect the actual administered dose [220]. Rest images
are obtained 30 minutes to 1 hour after injection, and post
stress imaging is performed approximately 15-20 minutes
post-exercise, or 30-60 minutes post-pharmacologic stress [178].
Some centers have the patients drink 8oz. of whole milk about 15
minutes prior to imaging to promote tracer clearance from the
liver and gallbladder. This, however, increases the amount of
bowel activity.
The radiation exposure is approximately 11-18 mSv [156]. Other authors note a radiation dose for a rest-stress protocol (10 mCi rest/30mCi stress) of 11.4 mSv for Tc-99m-sestaimibi and 9.3 mSv for Tc-99m-tetrofosmin [178]. A PA CXR has an effective dose of 0.02 mSv, a mammogram a dose of 0.4 mSv, and the average annual background exposure is 3 mSv [167]. Therefore, a SPECT MPI exam is the equivalent of at least 3 to 4 years of natural background radiation exposure [169]. It is estimated that for a 60 year old patient undergoing rest-stress MPI that approximately 8-9 future cancers per 10,000 scans would result from the radiation exposure [167]. Since the lifetime risk for cancer is 1 in 2 for men, and 1 in 3 for women, the estimated increased lifetime cancer risk from the exam would be less than 1% for both men and women [167]. However, the Quebec Medicare data for survivors of acute MI indicated that the cummulative effects of diagnostic imaging (nuclear perfusion and coronary angiography) and fluoroscopically guided coronary interventions resulted in a detectable increase in the incidence of malignancy at a rate of 3% over a 5 year period for every 10 mSv of radiaiton exposure [170,171]. A study evaluating DNA damage following CTA found that patients with dose levels above 7.5 mSv showed significant DNA damage on both proteomic and genomic analyses, and apoptosis [224]. However, most patients did not have detectable residual DNA damage 2 hours after exposure demonstrating the effectiveness of the body's repair mechanisms (most patients repair double stranded DNA damage to baseline levels within 24 hours of testing) [224]. However, in a minority, changes were discernable up to 1 month following the exposure (although the number of cells with residual damage was very small < 1%) [224]. Newer image reconstruction methods using ordered-subset expectation maximization with resolution recovery (OSEM-RR) can be used to lower the administered dose without compromising image quality [196]. The ASNC recommends using a protocol and administered activities that will result in radiation exposure as low as reasonably achievable (ALARA) for each patient, with radiation effective dose of less than or equal to 9 mSv in at least 50% of studies [223].Marked dose reduction can be accomplished using a solid state camera system for image acquisition [237]. The lowest possible total radiation dose from SPECT MPI (approximately 1 mSv) can be accomplished by performing stress only imaging with a solid state camera [237].
Drawbacks of the same day rest-stress protocol are that it
provides less than ideal stress defect contrast due to resting
background activity that may "shine-through" into the stress
images and decrease the apparent size and severeity of a stress
perfusion defect [178,236]. It was generally believed that
achieving a stress/rest radiopharmaceutical ratio of greater
than or equal to 3 obviates this concern [178]. However, in the
EXXERT study, ischemia was found to be present in approximately
30% more patients undergoing a multi-day exam compared to those
undergoing same day rest-stress imaging [236]. These authors
suggest that a higher stress to rest count ratio of 4:1, or a
longer time delay between exams (4 hours), should be used [236].
Another drawback is the relatively low rest dose that can result
in suboptimal count density with resultant poor image quality
and associated artifacts [178]. Moreover, comparison of the
low-dose rest to the high dose stress images may be problematic
if there are large differences in count density between the data
sets [178].
Tc-Sestamibi and Tc-tetrofosmin rest imaging have also been shown to underestimate the extent of viable tissue.
Two-day
Stress/Rest Protocol (Stress first imaging)
In routine clinical practice, up to 60-70% of appropriately
indicated MPI exams demonstrate normal perfusion [190,192].
Stress first myocardial perfusion imaging can shorten the exam
time, reduce costs, and decrease patient radiation exposure by
30-60% because the rest study would not be necessary if stress
study was normal [189,190,192]. Studies have shown a high
survival rate and similar clinical outcomes in patients with a
normal stress-only exam (99.3% for stress only compared to 99.2%
for rest-stress) [175,189,190,192]. Ideally, candidates for
stress first imaging would be non-obese patients with low to
intermediate likelihood for CAD, interpretable ECGs, regular
heart rates to permit gating, no history of prior MI and/or
CABG, and the availability of attenuation correction (when
performing stress imaging first, the use of attenuation
correction can enhance the confidence for a normal exam)
[189,192]. In one study, of stress first imaging, 58% of
patients had abnormal stress images- but attenuation correction
allowed reclassification of 83% of these ptients normal
stressing the importance of attenuation correction [193]. Prone,
in addtion to supine imaging, can also be used to aid in the
evaluation of suspected attenuation related perfusion defects
[190]. Factors associated with unsuccessful stress first imaging
include age over 65 years, diabetes, typical chest pain, CHF,
abnormal ECG, male gender, and documented CAD [189].
The two day exam provides optimal defect contrast with minimal
background activity. Because the dose used for the two exams is
the same, the spatial resoltuion, attenuation artifacts, and
Comptom scatter will be relatively similar [178]. A 2-day
protocol is particularly well-suited for obese patients and
patients in whom attenuation artifacts are anticipated [178].
The effective radiation dose using the 2-day protocol is 14.8
mSv for sestamibi and 11.6 bmSv for tetrofosmin (based on an
injection dose of 25 mCi for each exam) [178].
Dual
Isotope Scanning
In this protocol a rest thallium study (2.5-3.5 mCi) is done first so that there is no interference from scattered technetium photons. This approach eliminates the delay between rest and stress imaging and improves patient throughput. If Thallium is injected the day before, images will reflect 24 hour redistribution (myocardial viability). Delayed thallium imaging at 24 hours using the dual isotope exam will detect reversible defects in an additional 17% of patients. [5]
A stress Tc-Sestamibi or Tc-Tetrofosmin study is then performed using 20-30 mCi of the agent. No wait is required between the rest and stress study as the Technetium gamma energy is higher than the energy imaged for Thallium. A drawback of this technique is that because of the resolution differences between the two isotopes, it is difficult to directly compare the images, particularly with subtle abnormalities [178]. Patient radiation exposure is also higher [119]- effective dose is about 22-30 mSv [123,156,167,178].
Quantitative
analysis:
A polar map is available for quantitative analysis of the images. The distance weighted map is best for determining defect location as every ring in the polar map is the same width. The volume weighted map is best for determining defect size (extent and severity), as it makes the 2-dimensional area of a defect equal to its relative 3-dimensional volume.
The summed stress score (SSS) is a commonly used technique that
combines extent and severity of perfusion abnormalities (defined
from a sex-specific normal database) into a single measure and
it has been shown to provide risk stratificaiton
[64]. Post stress and and rest
perfusion images are scored using a either a 17 or 20 segment,
5-point model (0= normal; 1= mildly reduced uptake; 2=
moderately reduced uptake; 3= severely reduced uptake; 4=absent
uptake) [58,180]. Segmental uptake scores can then be added to
produce a summed stress (SSS) and rest scores (SRS). The summed
difference score (SDS) represents the difference between the SSS
and SRS [58]. A segmental defect is considered fixed if the
uptake score remains fixed between the stress and rest exams,
mildly reversible if it increases by 1 point, moderately
reversible if increases by 2 points,
and severely reversible if it increases by 3 points [58]. A SDS
score of less than 2 is considered normal with no evidence of
ischemia [64]. A SDS of 4 to 7 indicates mild-to-moderate
reversibility and a score of greater than 7 to 8 indicates
severe reversibility [58]. Many investigators consider a SSS
< 4 a negative exam [146]. A SSS of 4-8 is associated with an
event rate of 1-3% [144]. More extensive and severe
abnormalities, encumbering 10% of more of the myocardium, are
associated with up to 5% annual cardiac event rates [144].
The SRS and SDS can also provide prognostic information
regarding patient survival and risk for MI [102,141]. Patients
with normal LVEF's, but a SRS of greater than 6 have a poor
prognosis (2 fold decrease in survival rate) similar to patients
with a reduced LVEF (less than 45%) [102]. To convert the summed
stress score based on a 17 segment scoring system to a %
abnormal myocardium, the summed stress score would be divided by
68 (x100)- this can also be used for the summed difference score
to represent the % ischemic myocardium [187]. Patients with
>/= 10% ischemic myocardium are high risk patients with
nearly 5% annual cardiac death or MI risk [187]. Patients with
less than 10% ischemic myocardium are candidates for an initial
strategy of intensive medical and lifestyle intervention with
deferred revascularization [187].
For patients with moderate to severe LV dysfunction (cardiomyopathy/CHF patients), those with a SSS > 8 are two times more likely to die from a cardiac event than those with a SSS = 8 [204]. The cummulative 5 year survival has been reported to be 85.6% and 67.3% in patients with SSS = 8 and > 8, respectively [204.
Gating- Wall motion and
LVEF assessment
In non-gated SPECT imaging one projection image is acquired at each angular step along the acquisition orbit [82]. With gated SPECT, data acquired during each angular step is further subdivided into a specific phase of the cardiac cycle (typically 8 or 16 phases) based upon the R-R interval [82]. 16 phase data sets are required for diastolic function assessment, but can suffer from low counts [82]. Summing the data from the individual phases will produce a standard SPECT image [82], but ventricular function data can also be assessed by review of the gated data set. Gated imaging data can add important independent and incremental prognostic information to the data obtained from the perfusion exam [52,74]. Irregular arrhythmias such as atrial fibrillation and ventricular ectopy produce "flickering" in the rotating summed projection cine images [151]. This is due to the back-projection of varying data indicating count inconsistencies have occurred at different projection images [151]. On the other hand, regular arrhythmias such as a tachy/brady arrhythmia are not associated with "flickering" and may be overlooked [151]. For patients with severe arrhythmias (particularly atrial fibrillation) it is recommended that myocardial perfusion SPECT imaging NOT be performed with ECG gating (i.e.: these patients should have a non-gated SPECT exam) due to an apparent worsening of the perfusion pattern on summed gated images [127].
Wall motion analysis:
Gated studies can be used to assess regional wall motion and
left ventricular ejection fraction when viewed in a cine
display. Gated exams improve the diagnostic accuracy and
specificity [57] of SPECT imaging by detecting artifacts
secondary to soft tissue attenuation. The gated LVEF also
provides information regarding myocardial systolic function and
prognosis. Post exercise wall motion abnormalities add
significant incremental value over stress myocardial perfusion
alone for the identification of severe CAD [57]. Both wall
motion and wall thickening should be analyzed on gated imaging.
Wall motion is scored with a 6-point system: 0- normal; 1- mild
(equivocal) hypokinesis; 2-
moderate hypokinesis; 3- severe hypokinesis; 4- akinesis;
and 5- dyskinesis [82]. Wall
thickening is scored with a 4 point system from 0 (normal) to 3
(absent) [82]. The most common cause of discordance between wall
motion and wall thickening is found in patients who have
undergone bypass surgery - in these caseds there is abnormal
septal wall motion with peserved thickening [203]. Similar
discordance between wall motion and wall thickening can also
occur in LBBB, where preserved thickening with abnormal septal
wall motion is a normal variant [203]. Another point to remember
is that wall motion does not always indicate viability [203].
Normal wall motion of an abnormally perfused segment that does
not thicken could be associated with passive inward motion of a
non-viable region (tethering), due to hypercontractility of
adjacent non-infarcted segments [203].
The study requires performing a gated acquisition during each
of the planar projections. Optimally ECG gating is performed
using eight to 16 frames for the cardiac cycle. The images
obtained for interpretation are sharper than non-gated images
and can sometimes increase conspicuity of a perfusion defect.
Both wall motion and wall thickening can be evaluated on gated
cardiac exams. However, changes in heart rate can result in
temporal blurring- in other words- mixing of counts from
adjacent frames [51]. To minimize temporal blurring, a beat
rejection window is set by specifying the acceptable deviation
of each R-R interval from the expected value (a 20% window has
historically been applied) [51]. Wall motion is evaluated by
measuring the excursion of the ventricular endocardial
surface, while wall thickening is evaluated by assessing changes
in regional myocardial counts [34]. On gated images wall
thickening appears as an increase in myocardial intensity
(brightness) from diastole to systole. "Flashing" or
"flickering" on review of the projection images from a gated
SPECT exam is indicative of rejected beats (generally due to
arrhythmias or changes in heart rate during image acquisition)
which results in variation in count density in the projection
images [96]. Other authoirs state that the flicklering is the
result of count drop-off in the latter frames of the cardiac
cycle because of inclusion of beats that have a shorter R-R
interval than that at baseline, but still fall within a
prespecified acceptance window [188]. Whatever the etiology, the
end result is the presence of streak artifacts in the tomographic images [96]. This gating
error can affect the veracity of SPECT-derived functrional
parameters- including LVEF, wall thickening, and mechanical
dyssynchrony analysis [188]. Some authors suggest that a
nongated SPECT acquisition is mandatory to reliably assess
myocardial perfusion in patients with atrial fibrillation [127].
Systolic images have significantly greater quantitative recovery of absolute myocardial activity due to less partial volume loss of the thicker systolic LV wall compared to diastolic thickness [219]. If a perfusion defect is present on stress images and the associated wall is seen to thicken (ie: brighten) during systole, one can predict that this represents an area containing viable myocardial tissue. If no thickening is observed, the finding may represent an area of infarction (scar), however, one cannot completely exclude the presence of viable myocardium (hibernating myocardium). If there is insufficient counts from the area of the defect, viable myocardium may not be properly identified even on a gated exam. Hence, areas of hibernating myocardium may be akinetic or dyskinetic with no evidence of thickening, but will recover function following revascularization. False positive gated exams for infarction have also been described in patients with cardiomyopathies and in valvular heart disease in which non-ischemic perfusion defects and wall motion abnormalities may be seen [13].
Gated SPECT during the first hour after exercise is able to assess post-stress cardiac function [26]. Reversible perfusion defects may display post-stress stunning (transient myocardial contractile dysfunction/wall motion abnormality) in areas of ischemia [26]. The incidence and magnitude of regional wall motion abnormalities are related to the severity of ischemia [39]. The time course for resolution of ischemic post-stress wall motion abnormalities can range from immediate to up to 2 hours [32].
In patients with multivessel coronary artery disease, the degree of ischemia can be underestimated because of a relatively balanced global left ventricular hypoperfusion resulting in a normal appearing scan [32]. Post-stress regional wall motion abnormalities detected on the gated exam may be the only indicator of severe and extensive coronary disease and provides incremental diagnostic information over perfusion alone [26]. Wall motion abnormalities identified on gated SPECT imaging provide additional prognostic information [112]. Reversible wall motion abnormalities between stress and rest images are indicative of severe coronary artery disease and have been shown to be a powerful predictor of future cardiac events [65]. Patients with abnormal wall motion on gated SPECT images have an annual event rate up to 6.1%, compared to 1.6% for patients with normal wall motion [52]. Even in patients with preserved ejection fraction, the presence of wall motion abnormalities has been found to be associated with an increased risk for cardiac death (increased to 1.6% from 0.5% if no regional wall motion abnormality was identified) [112]. The cardiac death rate increased to 2.2% when the exam also demonstrated evidence of ischemia [112].
Breast attenuation, diaphragmatic attenuation, and apical thinning can all produce fixed perfusion abnormalities. On gated images, these defects will generally demonstrate normal wall thickening which would not be identified in an area of scarring. Another technical limitation of gated imaging is overestimation of viable myocardium. Approximately 5% of myocardial infarctions are seen to thicken during systole on gated images. This may occur because a small infarct is "pulled in" by adjacent normal myocardium, or the MI may be non-transmural. False positive gated exams for infarction have been described in patients with cardiomyopathies or valvular heart disease in which non-ischemic perfusion defects and wall motion abnormalities may be seen [13].
Abnormal reduced or paradoxical septal motion with normal wall thickening is a common finding on gated SPECT images after coronary artery bypass surgery [34,82,191]. This finding is felt to be related to exaggerated systolic anteromedial cardiac translation [34]. As a result of this translocation, lateral wall function is often overestimated [34]. The exaggerated cardiac mobility is thought to be related to sternotomy and pericardiotomy [34]. The paradoxical septal motion does not appear to affect or impact LV mechannical dyssynchrony indices measured from gated SPECT [191]. Discordant septal thickening with abnormal wall motion can also be seen in patients with LBBB [82].
Ejection fraction:
The cardiac ejection fraction can be calculated from the gated SPECT data and provides incremental prognostic information for risk stratification. An LVEF < 45% and LV end-systolic volume > 70 mL provide additional risk stratification beyond clinical variables and the MPI exam findings [221]. There are several software programs which can generate the EF and all have roughly between 70-85% correlation with gated blood pool imaging determined ejection fractions [23]. With serial measurements the calculated LVEF is very reproducible [43] and has a mean variability of about 5% (+/- 3.5%) (higher variability is associated with low count examinations) [38,95]. The LVEF also has good correlation with cine MR determined values [71]. In patients with stress induced ischemia, left ventricular function may be temporarily impaired (stunned myocardium) [43,61]. Global LVEF is generally not impaired until at least 25% of the LV myocardium is ischemic [39,65]. Global LVEF impairment is also more commonly associated with anterior (LAD) ischemia [39]. Gated SPECT imaging started soon/early after exercise is likely more sensitive to the detection of post-stress stunning than conventional delayed imaging- particularly in patients with single-vessel disease [61,107,215]. In patients with "balanced" multivessel ischemia perfusion images alone may significantly underestimate the extent of CAD and an abnormal post-stress LVEF may be the only indication of underlying CAD [51,82]. A greater than 5% decrease in LVEF is an indicator of multi-vessel CAD with a sensitivity and specificity of 52% and 83%, respectively [100,137]. Even following pharmacologic stress with adenosine, a decrease in EF between rest and stress of = 5% has a sensitivity and specificity of 60% and 77%, respectively, for mutlvessel CAD [138]. Myocardial stunning with decreased LVEF and wall abnormalities can also be seen on gated images following both adenosine and dipyridamole stress and is also an indicator of severe CAD [80,81].
The presence of ischemia on SPECT imaging places the patient at increased risk for subsequent ischemic event, but does not correlate with survival [94]. For prognostic information regarding survival, the post-stress ejection fraction is an important determinant for the risk of cardiac death [14,82,93]. Patients with a post-stress ejection fraction of less than 30% are at an increased risk for cardiac death, regardless of the amount of ischemia identified [14]. Other authors indicate that a post-stress ejection fraction of less than 45% is associated with an elevated risk of major adverse cardiac events [49,51,57]. A greater than 5% decrease in LVEF between rest and stress has been shown to be an independent predictor for the need for early revascularization [65]. However, ejection fraction alone is not the sole determinant of the potential benefit of revascularization [93]. Although patients with ischemia benefit from revasularization (with increasing survival benefit with increasing amounts of ischemia), the combination of ischemia AND a decreased ejection fraction indicates patients that will benefit the most from revascularization [93]. This data has significant implications regarding the management of CAD patients- particularly given recent data that suggests percutaneous coronary intervention does not decrease the rate of myocardial infaction or mortality other than in the acute coronary syndrome setting (i.e.- in the setting of an acute coronary syndrome, invasive coronary revascularization has a morbidity and mortality benefit) [93]. In fact, patients with drug eluting stents are at risk for acute thrombosis with an incidence up to 1.3% at 9 months and a 45% case fatality rate [93].
Following myocardial infarct: In the early phases following acute MI, left ventricular dysfunction is an independent factor for risk stratification [36]. In the post-MI period, a gated LVEF of less than 40% has been associated with an increased risk for subsequent cardiac event [33]. Generally, the relative risk of death or non-fatal MI doubles for every 10% decrease in LVEF [50].
Unfortunately, early post MI left ventricular function may be impaired [36]. Dysfunctional, but viable, stunned myocardium will gradually return to normal function, and hibernating myocardium will demonstrate improve function following revascularization [36]. Assessment for contractile reserve in areas of hibernating myocardium can be performed using low dose dobutamine (LDD) echocardiography or LDD SPECT imaging (see discussion in Pharmocologic stress imaging section). In patients with dilated cardiomyopathy, an increase in SPECT LVEF following low dose dobutamine infusion can be used to predict improvement in cardiac function and heart failure symptoms after institution of beta-blocker therapy [59].
Proper ECG triggering is crucial for accurate LVEF determination- there should be a regular cardiac sinus rhythm and exclusive R-wave detection [66]. Gating errors can affect wall motion analysis and LVEF determination [46]. In patients with arrhythmias, EF fluctuations and wall thickening discordance may occur [51].The mixing of R- and T-wave triggered beats leads to a fall in the calculated LVEF [66]. With very tall T waves, the R-T phase (systole) and the T-R phase (predominantly diastole) are regarded as two cardiac cycles [46]. When the time-volume curve is generated by summation of these phases- which produces essentially a flat line [46]. Repositioning the ECG leads to increase the amplitude of the R wave and decrease the T wave can correct this error [46]. Patient motion can also affect the calculated LVEF [66]. Even one pixel of displacement can result in a change in an absolute change in LVEF of 5% +/- 4% [66].
Underlying tachycardia can also affect the LVEF [151]. Tachycardia results in a shortened diastolic interval and hence a greater proportion of counts obtained from the systolic frames [151]. Systolic phase images tend to have a pattern of thickened LV walls, a contracted LV chamber, and a decreased end-diastolic LV volume [151]. Most software packages calculate LVEF using endocardial edge tracking- as a result, an abnormally shortened diastolic (or prolonged systolic)phase can result in underestimation of end-diastolic volume and consequently a low LVEF [151].
A limitation of software for gated SPECT is overestimation of LVEF [37] and underestimation of LV end-systolic volumes in small ventricles, especially in patients with hyperdynamic LV function (the small heart error). The inaccuracy in measurements is a result of the low spatial resolution (about 15mm) of SPECT imaging which limits effective delineation of the endocardial surface used for LVEF determination [37,51]. This is particularly a problem at end-systole because the LV is at its smallest and the endocardial edges are at their closest points [23]. In fact, the endocardial edges may appear to overlap [23]. Incorrect determination of the position of the edge of the endocardial surface results in an underestimation of end-systolic volume, which results in an overestimation of the LVEF [23]. End-diastolic volume is also markedly underestimated when less than 37 mL [23]. Programs which do not rely on edge detection may provide more accurate LVEF determinations for patients with small ventricles [23]. Arrhythmias do not seem to significantly affect ejection fraction or ventricular volume determinations [25], although other authors state that LVEF determination can be affected by arrhythmias [51]- specifically a falsely low ejection fraction [96].
Ventricular volumes:
Using gated SPECT software the left ventricular end diastolic volume (normal less than 120 mL) can also be calculated and is generally within 15% of the actual end-diastolic volume when the volume is greater than 74 mL [23].
End-systolic LV volume is an important predictive factor of long-term prognosis in patients with ischemic heart disease [60]. An end-systolic volume of greater than 70 mL places patients in a high risk category [57,221]. An increase in ESV of greater than 5 mL between stress and rest is also an indicator of multi-vessel CAD [100].
The presence of an increased end-diastolic volume also carries additional prognostic information. LV enlargement significantly increases the mortality rate in patients with acute MI- particularly when there is coexisting myocardial dysfunction [50]. Patients with an LVEF lower than 40% in whom LV expansion develops, have a significantly higher mortality rate compared with those who do not [50]. An increase in EDV of greater than 5 mL between stress and rest is also an indicator of multi-vessel CAD with a sensitivity and specificity of 66% and 87%, respectively [100,137]. Other authors have suggested that even with pharmacologic stress with adenosine changes in EDV and ESV may also indicated multivessel CAD [138]. An increase in EDV greater than 6mL or ESV of greater than 6mL between rest and pharmacologic stress had the following sensitivities and specificities for multi-vessel CAD- change EDV 60% and 74%, respectively; change ESV 81% and 77%, respectively [138].
Following an MI, left ventricular remodelling refers to changes in LV size, shape, and thickness involving the infarcted, as well as remote normal regions of the left ventricle [140]. A combination of LV dilatation and hypertrophy of residual remote normal myocardium is a manifestation of LV remodeling [140]. Progressive enlargement (or remodeling) of the left ventricle can develop during the first months after an acute MI and this has a negative influence on long-term prognosis [60].
Diastolic dysfunction:
Diastolic dysfunction results in ineffective left atrial
emptying and left ventricular filling, reduces the ability to
augment cardiac output with exercise, increases pulmonary
pressure, and results in symptoms and fluid retention [234].
Diastolic relaxation and filling appear to be altered by
ischemia [234]. Diastolic dysfunction precedes the onset of
systolic dysfunction and takes longer to resolve [234]. The
presence of diastolic dysfunction has been shown to be
associated with non-obstructive coronary atherosclerosis at
angiography [233]. Additionally, the presence of diastolic
dysfunction in patients with CAD increases morbidity and
mortality 4-fold [125]. Patients with diastolic dysfunction can
have normal ejection fractions [125]. Diastolic filling
parameters can be determined from gated SPECT exams using 16
frame acquisitions [125]. Abnormalities in peak filling rate
(PFR), time to peak filling rate (TPFR- as diastolic dysfunction
worsens, the TPFR increases), and filling rate during the first
third of diastole (1/3FR) can all provide information regarding
the presence of elevated left ventricular end-diastolic pressure
(greater than 18 mm HG is a major predictor of LV diastolic
dysfunction) [125]. A PFR = 2.57 EDV/s, a 1/3FR = 1.52 EDV/s, or
a TPFR = 161 ms are indicative of LVEDP of greater than 18 mmHg
[125]. Ventricular filling parameters cannot be used for
patients with irregular heart rates [125]. 233
Phase analysis:
Cardiac resynchronization therapy (CRT- in which both the left and right ventricles are paced - biventricular pacing) represents an effective treatment option in patients with moderate-to-severe drug-refractory heart failure [154]. CRT has been shown to reverse ventricular remodelling, improve mechanical contractility/synchrony, left ventricular (LV) function, and reduce hospitalization and death in patients with advanced/chronic/drug refractory CHF and LBBB [114,143,207]. CRT can improve clinical manifestations and quality of life, reduce hospitalizations for CHF, reduce complications, and risk of death (increase survival) [115,116]. CRT is recommended for heart failure patients with NYHA class III-IV symptoms that are refractory to medical therapy that meet specific criteria [172].Traditional selection criteria for CRT are an LVEF < 35% and a widened QRS complex (>120 ms) [115,116]. Unfortunately, between 20-50% of patients who meet the QRS criteria do not respond to CRT [114, 116,129]. This may be because the QRS duration is a marker of electrical dyssynchrony, however, mechanical dyssynchrony is a more powerful predictor of response to CRT [120] and there is only moderate correlation between LV electrical and mechanical dyssynchrony [207]. Mechanical dyssynchrony can also be observed in patients with normal QRS duration [122]. In one study, almost 29% of patients with only mild-to-moderate LV dysfunction (LVEF 35-50%) and a QRS duration of less than 120 msec were found to have significant mechanical dyssynchrony by phase analysis [148]. In another study of patients with non-ischemic cardiomyopathy, a LVEF between 35-50%, and a QRS < 150 ms, the presence of post stress mechanical dyssynchrony on MPS imaging was associated with an increased risk for all-cause mortality [206].
Implantable cardiac defibrillators (ICD) are used in patients at risk for sudden cardiac death and include survivors of sustained ventricular tachycardia or ventricular fibrillation [154]. Patients with prior MI and depressed LV systolic function (LVEF<30-40%) have also been treated with ICD's to improve overall survival [154]. LV dyssynchrony is also associated with an increased risk of adverse cardiovascular events in ICD recipients and biventricular pacer-defibrillator devices may be more appropriate in these patients [154].
Phase image analysis from gated cardiac examinations can be
used to evaluate for the presence of LV mechanical dyssynchrony (GSPECT) and is a largely
automated process [116,121]. Phase analysis is based on the
partial volume effect which states that the LV regional maximal
counts on SPECT MPI images are nearly proportional to the
myocardial wall thickness of the same region (the brightening is
due to the fact that objects show variable count density in
proportion to their thickness, when that thickness is less than
twice the resolution of the imaging device, as measured by full
width at half maximum on the imaging systems response curve-
end-diastolic LV wall thickness falls into the size range of
objects subject to this effect) [172,229]. Variation of regional
myocardial counts reflects thickening of each segment over the
entire cardiac cycle [221]. Phase images are color coded
displays of the relative timing of the onset of contraction in
each cardiac pixel and represent the ventricular contraction
sequence [117]. Phase analysis therefore provides information
regarding how uniform or inhomogeneous the left ventricle is
contracting [121]. Since the normal ventricle contracts in a
regular synchronous fashion, areas of unusually early or late
contraction (dyssynchrony) can be seen visually [229]. Phase
analysis can detect phase delays using gated SPECT MPI data
acquired with either 8 or 16 frames/cycle [172]. Phase analysis
is larged automatic and has been shown to have high
reproducibility and high repeatability [172,207]. It is best to
determine phase indices from higher dose exams (such as post
stress images), as phase analysis indices performed on low-dose
tracer studies demonstrate more variation [177]. The lower
signal to noise ratio is likely one of the main reasons for this
problem. Up to 9-13% of patients can be falsely labeled as havng
mechanical dyssynchrony when using low dose rest exams for
evaluation [177]. Paradoxical septal
motion on gated images that is commonly seen following CABG does
not appear to affect or impact LV mechannical dyssynchrony
indices measured from gated SPECT [191].
Phase histiogram, phase standard deviation, histiogram
bandwidth, and phase entropy are the most significant
quantitative indices of LV dyssynchrony [238].
A phase histiogram curve is
generated with the x-axis representing the time (or phase angle)
and the y-axis representing the portion, as a percentage, of the
myocardial wall that starts to contract as a specific time
[121]. The term phase angle is used where end-diastole is
considered 0º (or 360º) and end-systole is approximately 180º-
hence 0º to 360º represents one cardiac cycle (R-R interval)
[117,121,148]. Ventricular pixels and atrial
pixels are typically 180º out of phase with each other [117].
Normally, the LV contracts in a coordinated way so that most
myocardial segments have nearly the same phase (for normal
ventricles- septal pixels have the
earliest phase, after which the rest of the ventricle shows a
fairly uniform phase as long as the His-Purkinje tissue is
functioning uniformly) [117,121]. Thus, the normal phase image
is close to a uniform distribution and the normal phase histiogram is narrow and highly peaked
[121,221].
The standard deviation of the phase distribution is calculated
(phase SD) and measures the extent of the deviation of the onset
of mechanical contraction in the LV and thus, the larger the SD
the more dyssynchronous the LV [238]. The normal phase SD range
is between 5.1 degrees and 31.4 degrees with a mean of 14.2
degrees in males and 11.8 degrees in females [238]. The phase
(or histiogram) bandwidth represents the phase range during
which 95% of the LV myocardial voxels initiate contraction
(range of phases/onsets of contraction encompassing 95% of the
phase distribution) [162,214,230,238]. As the LV mechanical dyssynchrony worsens, the phase standard
deviation (SD) and histiogram
bandwidth are expected to increase [122]. Phase entropy is a
measure of "disorder" showing a range between 0 and 1 (0%-100%)-
the more disordered the LV, the more dyssynchronous [238].
On phase analysis, preliminary data indicate that a response to
CRT can be predicted by a histiogram
bandwidth of more than 135º (sensitivity 70% and specificity
70%) and a phase SD of more than 43º (sensitivity and
specificity 74%) [121]. In another study using QGS software, a histiogram bandwidth cutoff of 72.5º
yielded a sensitivity of 83% and a specificity of 81% in
predicting a response to CRT (phase SD cutoff was
19.6º for a sensitivity of 83% and a specificity of
81%) [143]. Differences may exist between various software
packages (QGS vs Emory Toolbox) in
determining appropriate cut off values [143]. Changes in
dyssynchrony parameters occur immediately following CRT
implantation and may predict long-term LV reverse remodeling
[238]. Similarly, improvement in LV dyssynchrony at 6 months
post-CRT is associated with clinical outcomes [238].
Gating errors can affect the accuracy of mechanical
dyssynchrony evaluation by SPECT producing a spurious decrease
in phase SD due to count drop-off in frames late in the cardiac
cycle [188]. This likely alters the fit of the first sinusoidal
harmonic to this perceived trough at the end of the cardiac
cycle- the magnitude of which is proportional to that of the
drop off [188]. This effect is magnified at longer phase SD's
[188]. Using a narrow beat acceptance window can help to avoid
gating error artifacts, but this would extend the duration of
the exam or require retrospective (list-mode) gating [188].
On phase analysis, fixed perfusion defects can be detrimental to the quantification of dyssynchrony as these regions will not be shown to contract [130]. However, this may not be clinically important as studies have shown an inverse relationship between the degree of myocardial perfusion abnormalities and response to CRT (i.e.: patients with more extensive fixed perfusion abnormalities- or areas of scarring- are less likely to respond to CRT) [129,143,160,174,214]. In one PET study, a scar burden of greater than 15% was associated with a poor response to treatment [160]. Delayed contrast MR studies have also demonstrated a scar volume of 15% or more to be associated with lack of response to CRT [162]. Other studies have suggested that posterolateral or lateral scar location (sites commonly used for the position of the LV pacing lead) also correlate with a worse CRT response [160,162,172]. The rationale for this is that it is more difficult to resynchronize scar than viable myocardium, particularly when the scar is located in the late-activated region of the LV where LV pacing usually occurs in CRT systems [162]. Therefore, SPECT imaging has the ability to detect not only LV dyssynchrony, but also the extent and location of scar tissue [143]. Patients that have LV pacemaker leads positioned within non-viable myocardial areas are also less likely to demonstrate a response to resynchronization therapy [160,214].
Finally, the location of the pacing site within the LV has been
shown to influence the degree of functional improvement in CRT
[143,149]. Patients with the LV lead positioned outside the
region of latest mechanical activation benefit less from CRT
than patients with the lead positioned in the area of latest LV
activation [143,172,174]. A new development in phase analysis
has been the quantification of regional mechanical activation
[172]. A 7-segment model is used to divide the phase polar map
into apex, anterior, lateral, inferolateral, inferior, septal,
and anterospetal regions [172]. The region with the largest mean
phase is the site of latest mechanical activation [172].
Placement of the pacer lead in this region (latest mechanical
activation) has been shown to be associated with a better
response to CRT [172].
Change in LVEF following a low-dose dobutamine infusion can be
used to evaluate myocardial contractile reserve [176]. Patients
with higher contractile reserve(improvement of equal to or
greater than 6% in LVEF following a low dose dobutamine
infusion) are more likely to demonstrate improvement in LVEF
following resynchronization therapy and improved event-free
survival [176]. Overall, myocardial contractile reserve may be a
better predictor of functional improvement following
resynchronization therapy than baseline dyssynchrony as measured
by the gated radionuclide exam (MUGA) [176].
Overall, the success or failure of CRT depends on several
variables [181]. At a minimum they appear to include the
presence and the magnitude of LV dyssynchrony, the presence and
extent of areas of LV infarction (in particular the target
segment for pacing), and the successful delivery of a pacing
electrode to a site with markedly delayed contraction that has
adequate contractile reserve [181].Patients with persistent
episodes of VT following CRT have been shown to have more
non-viable myocardial segments and are more likely to have
non-viable segments in the region of the LV lead position [202].
Various cut-offs for increased VT risk following CRT have been
described including less than 15.5 viable segments and a scar
mass of greater than 22% [202].
Using delayed enhanced cardiac MR imaging, patients that demonstrate posterolateral scar tissue seem to be less likely to respond to CRT than those without scarring in that area (14% vs 81%, respectively) [129].
SPECT imaging can be used to monitor response to CRT
intervention. Measuring LV volumes prior to and following CRT
may provide prognostic information [155]. Studies have proposed
that a 15% reduction in end-systolic volume indicates a positive
response to CRT [155]. Serial followup
phase analysis exams can be performed following institution of
therapy to evaluate for improved LV function [157]. There is
good repeatability of functional parameters- especially when
serial studies are processed side-by-side [157].
In patients with ICD's, the severity of mechanical dyssynchrony
by phase analysis is associated with an increased risk of ICD
shock and cardiac death [172].
In patients presenting for the evaluation of known or suspected
CAD, LV mechanical dyssynchrony by phase analysis is a strong
independent predictor of major adverse cardiovascular events
[221,232]. Phase analysis from gated SPECT imaging has also been
shown to predict risk for cardiac death in patients with LV
dysfunction (LVEF < 50%) [210,211]. When an abnormal phase SD
cutoff of 40º was used, composite cardiac events occured in
12.4% of patients with an abnormal phase SD, but only 3.5% of
patients with normal phase SD [210,211].
Increased global and territorial dyssynchrony can be seen on
early (5-10 minute) post stress imaging compared to rest images
in patients with multivessel CAD and may reflect the effects of
myocardial stunning [213].
Exercise
First-pass LVEF
Technetium agent first pass LVEF determination requires the use of a multicrystal camera. In patients with multivessel disease (particularly 3 vessel disease), the perfusion study may underestimate the severity coronary artery disease (balanced ischemia). These patients, however, often demonstrate a significant drop in LVEF with exercise. A normal exercise LVEF does not exclude the presence of CAD, but it is associated with a better prognosis in patients with CAD. Patients with an abnormal exercise LVEF and known CAD may benefit more from surgical intervention (CABG,PTCA) than from continued medical therapy.
First pass LVEF seems to be the single most important predictor for the risk of cardiac events in patients with known coronary artery disease. An exercise LVEF of 20-40% is associated with a 15% one year cardiac mortality rate, while an exercise LVEF < 20% is associated with a 50% one year cardiac mortality rate.Determination of first pass LVEF in association with pharmacologic stress has not been shown to add clinical information to the perfusion exam [6].
Chamberdilatation
with stress
The trasient ischemic dilatation
(TID) ratio is calculated as the ratio of the ungated LV cavity volume after stress
and at rest [82]. As with thallium imaging, transient LV
dilatation is a marker of severe and extensive coronary artery
disease [70]. Although TID is highly specific for the presence
of severe CAD, the reported sensitivity is more variable - from
23-71% [128]. Patients with TID have a poor prognosis with
cardiac event rates from 11% to 60% [128]. However, several
authors suggest that for patients with otherwise normal
perfusion exams (normal perfusion, normal LVEF, and normal LV
volume), TID may not indicate a worse prognosis or increased
risk for CAD (i.e.: to carry predictive accuracy, TID should be
observed in conjunction with other perfusion
abnormalities)[165,173,200,209,221]. However, for patients with
high risk comorbidities such as underlying diabetes or known
CAD, TID still carries an increased risk for cardiac death or MI
even when the perfusion scan is otherwise normal [200]. In
another study, about 40% of diabetic patients with TID did not
have severe CAD on angiography- this suggests that epicardial
CAD may not be the sole pathophysiologic process responsible for
TID in diabetic patients and that coronary microvascular disease
may play a role [194,200].
With technetium agents, stress imaging is delayed 30 to 60 minutes following completion of stress and therefore the left ventricle (LV) is unlikely to still be transiently dilated due to ischemia [185]. If post-stress left ventricular chamber dilatation is noted on delayed imaging, it is most likely a reflection of diffuse subendocardial ischemia/hypoperfusion (which produces apparent thinning of the ventricular wall on post stress imaging) caused by multivessel coronary artery disease, rather than true cavity dilatation and this is supported by echocardiography [70,128,185,194]. Temporary systolic dysfunction may also contribute to the apparent increased cavity size [76]. Left ventricular chamber dilatation may also be noted with pharmacologic stress and is associated with an increased risk for subsequent cardiac event (11% versus 2%) [10,72]. This most likely represents subendocardial ischemia or diffuse reduced subendocardial flow reserve [72].
The cutoff values for abnormal transient ischemic dilatation
vary throughout the literature [76,82].
When comparing exercise stress technetium images to rest either
rest technetium agent or thallium images, a TID ratio of greater
than 1.22 is considered abnormal [58,63,72]. Other authors
report an abnormal TID as equal to or above 1.19 for same day
rest/stress sestamibi imaging [182,194]. For adenosine stress
dual-isotope imaging a TID ratio of greater than 1.36 is
associated with a high sensitivity and specificity for severe
and extensive CAD (sensitivity 71%, specificity 86%) [63]. An
abnormal TID for same-day dual isotope imaging with dipyridamole
has been reported to be 1.27 and 1.4 for dobutamine [182].
The TID ratio may need to be adjusted for gender with one study suggesting an upper limit of normal for females as 1.31 and 1.18 for males [83]. A hypertensive response to exercise (BP systolic greater than 210 [men] or 190 [women]) has been reported to have a higher association with TID- even without other significant perfusion defects [110]. Although followup cath data was not available to consistently exclude the possibility of underlying CAD [110]. Left ventricular hypertrophy and diabetes may also be associated with a higher risk for TID- both of these disorders can cause coronary flow reserve abnormalities [128].
Interestingly, transient arrhythmias during image acquisition
can produce falsely elevated or reduced TID measurements and
some authors have shown that the TID ratio can vary with heart
rate [152]. The mechanism is postulated to be related to
diastolic shortening with high heart rates that
results in reduced intracardiac
flow- particularly in the subendocardial
regions [152]. At slower heart rates, the diastolic phase is
prolonged- creating optimal perfusion of the subendocardium [152]. Since TID is
calculated based upon differences in volumes that are derived
from count distributions- decreased subendocardial
perfusion during post-stress image acquisition can produce the
impression of increased TID [152]. Long-standing hypertension
has also been linked to the presence of TID [209]. This
condition results in a relative reduction in subendocardial
perfusion durng stress, possibly because of the increased
epicardial diastolic pressure required to perfuse the entire
width of the myocardium [209]. Apparent TID is also commonly
observed in patients with hypertrophic cardiomyopathy without
significant CAD during vasodilator stress (up to 50% of patients
on PET/CT) [228]. The finding of TID in these patients appears
to be related to abnormal regional subendocardial myocardial
perfusion, globally impaired vasodilator flow reserve, and
degree of hypertrophy [228]. Diabetes has also been described in
association with TID and otherwise normal perfusion- possibly
related to microvascular disease resulting in subendocardial
ischemia [209].
A planar LAO image immediately following exercise can be performed to assess true chamber size. If LV dilatation is present, there is a high association with multivessel CAD and high grade stenoses.
|
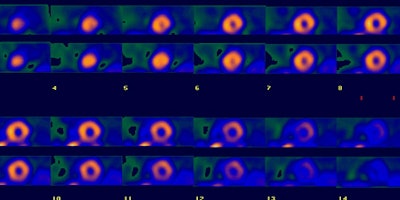
Multivessel coronary artery disease: The patient below underwent Tc-myoview SPECT imaging following pharmacologic stress with adenosine. The examination demonstrated a mild fixed perfusion abnormality in the inferolateral wall that was felt to be related to diaphragm attenuation. However, there was mild dilatation of the LV on stress imaging compared to the rest exam. At cardiac catheterization, the patient was shown to have three vessel disease. (Click image to view all slices) |
|
|
Pulmonary activity on
stress imaging
An increase in Tc-sestamibi lung uptake on stress imaging is a marker for severe underlying coronary artery disease or high grade stenosis (over 90%) of the proximal LAD [29,109]. A lung to heart ratio (LHR) of greater than 0.33 has been reported as suggestive of underlying severe CAD [29]. On early sestamibi SPECT images acquired early (15 minutes) after exercise, other authors report a LHR of greater than 0.44 to yield a sensitivity of 63%, and a specificity of 81% in identifying severe and extensive CAD [109]. Diffuse lung uptake can also be seen in smokers and in patients with inhalational pneumonitis [86].
Right ventricular increased uptake:
The criterion for increased right ventricular tracer uptake on stress images is an exercise right ventricular-left ventricular uptake ratio greater than 0.42 (normal is 0.24-0.36) or exercise right ventricular-rest right ventricular uptake ratio greater than 1.2 (i.e.: a greater than 20% increase from rest to exercise) [91]. Increased right ventricular activity can be seen in conditions which produce right ventricular hypertrophy such as COPD, pulmonary arterial hypertension, and congenital heart disease (atrial septal defect) [91]. In general, these patients will have increased uptake on both stress and rest images [91]. Increased right ventricular activity on stress imaging only is sensitive (90-93%), but not specific (30-49%) for severe left main or multivessel CAD [91]. In these cases, the etiology is likely related to a global reduction in LV uptake due to hypoperfusion with relative preservation of RV tracer uptake, as well as an increase in RV wall stress associated with LV ischemic dysfunction [91].
Prognostic
Value:
Risk stratification and prognostic assessment for the technetium cardiac perfusion agents is comparable [55].
Patients with
acute chest pain
In the evaluation of patients with chest pain of suspected cardiac origin, but no ECG evidence of cardiac ischemia, Tc-Sestamibi and Tc-Myoview rest imaging can provide diagnostic and prognostic information, as well as provide additiona risk assessment beyond that provided by clinical risk assessment alone [18,27,179]. The results of the SPECT exam can be instrumental in guiding patient management and limiting unnecessary invasive procedures [179]. Perfusion imaging in the acute setting is most useful in those patients with no history of prior MI or underlying cardiac disease [48,179]. The exam is less useful in patients with a history of prior MI in whom perfusion defects can be anticipated [18].
Patients injected in the ER, with evidence of a perfusion defect on the MIBI scan have a high likelihood for coronary artery disease or a myocardial infarction [9,56]. The size of the defect carries clinical implications as patients with larger defects have a worse long-term prognosis [56,179]. Patients with chest pain and a normal rest perfusion exam have a low likelihood for coronary artery disease and very low (less than 1%) 30 day cardiac event rate (the one-year event rate is also low - 3%) [9,56]. Based upon this data, patients could be risk stratified regarding their risk for actual cardiac event and potentially discharged [10]. Using a normal myocardial perfusion exam as a criterion NOT to admit patients can reduce total admissions and result in substantial cost savings (a 32% reduction in the odds of being admitted to the hospital to rule out MI) [27,35,92].
Additional investigators have also studied the effect of troponin levels in conjunction with MPS findings [99]. Low level elevations in cardiac troponin levels have been shown to identify patients at increased risk for future cardiovascular events [99]. However, when found to have normal MPS exams, these patients were shown to have a high-event free survival rate; whereas, an abnormal exam was associated with a 3-fold higher risk of all cause mortality and a 7-fold higher risk for 6 month cardiac events [99].
Tc-Myoview has also been used in this setting [27]. The exam has a sensitivity of 90% for acute myocardial infarction [27]. Patients with definitely normal exams have a very low cardiac event rate both during the hospital period and during short-term follow-up, with a high negative predictive value of 99% [27]. Unfortunately, many patients do have abnormalities on their studies (such as old areas of infarction) which decreases the specificity (60%) and the positive predictive value (12%) [27]. Between 2-5% of patients with normal scans may have underlying coronary artery disease that will require revascularization and further outpatient evaluation will be required in select patients [27].
A limitation of MPI is that a perfusion defect may represent acute ischemia, an acute infarct, or an old infarct- however, in each case the presence of a perfusion abnormality places the patient in a higher risk category, particularly if the patient was initially considered low risk [166]. Another limitation of acute MPI is that the sensitivity decreases as the symptom-free interval increases [166]. For instance, detection of CAD/abnormal MPI can be as high as 96% of patients injected during chest pain, but decreases to 57% of patients when imaged within 6 hours of their last symptoms, and decreases to 8% if imaging was delayed greater than 12 hours from symptoms [166]. Additional drawbacks of imaging patients with acute chest pain are the technical and logistical difficulties of imaging patients in the acute setting, the limited availability of imaging after normal hours, and agent preparation times- Sestamibi requires 30 minutes to prepare the dose and has a shelf life of only 6 hours (which would require preparation 4 times a day to have the dose routinely available on an emergent basis). Also- some infarcts will be missed- it has been shown that at least 3-5% of the left ventricle needs to be involved to visualize an area of hypoperfusion [56,92,166]. Generally, missed infarcts will be small, non-Q wave inferior infarcts which will usually have an uncomplicated course [56], however, these small infarcts could be detected with troponins [92]. In fact, up to 34% of chest pain patients with normal perfusion by SPECT imaging may have an acute coronary syndrome (such as unstable angina) [92].
Imaging the
Acute MI
The purpose of reperfusion therapy in patients with acute myocardial infacrtion is to achieve significant myocardial salvage and to limit the extent of irreversible tissue damage [20]. Salvaged myocardium is defined as the the difference between the initial area at risk and the final infarct size. Even a satisfactory angiographic result after PTCA does not always imply effective tissue reperfusion with consequent myocardial salvage [20]. Technetium based perfusion agents such as Sestamibi or Tetrofosmin can be injected in the emergency room to patients experiencing acute myocardial infarction. After initiation of reperfusion therapy and stabilization, images can be obtained which represent the extent of myocardium at risk at the time of infarction [50]. Subsequent images performed prior to discharge can be compared to the original exam and can assess the degree of myocardial salvage and viability [7,21].
There is excellent correlation between the size of histologic infarction and the measured region of absent perfusion on tomographic images. The absolute minimal activity (ie: the lowest pixel count) within an area of infarction, is strongly predictive of the eventual infarct size and the presence or absence of collateral flow. Although correlation with viability is reasonably high in the setting of acute infarction, cellular myocardial function does not immediately return to normal following revascularization and follow-up exams should be delayed for a period of time prior to reimaging [21]. Sestamibi uptake may not return to normal for up to 14 days following MI, reflecting metabolic derangements of tracer uptake or persistent microvascular dysfunction which limits the return of flow. The amount of salvaged myocardium is typically completely demonstrated by 7 days following revascularization on Tc-tetrofosmin imaging [21].
Another important factor in the evaluation of patients following emergent reperfusion for acute infarction is the resultant left ventricular ejection fraction (LVEF) [20]. Unfortunately, gated SPECT ejection fraction may not be accurate in the immediate post infarct period due to post-ischemic stunning and persistent contractile dysfunction [20]. The demonstration of contractile reserve within asynergic segments using dobutamine echocardiography (inotropic stimulation) can identify viable, but "stunned" myocardium [20]. The detection of any contractile reserve (even minimal wall motion) in the infarcted zone is an accurate predictor of increased LVEF during late follow-up [20].
Reverse redistribution -
Paradoxical perfusion pattern:
Reverse-redistribution or a paradoxical perfusion pattern
occurs when tracer uptake in a segment is greater at stress
imaging compared to the rest exam [183]. An area of
reverse-redistribution can be seen following PTCA in patients
with acute MI- indicating that the ability of myocytes to retain the tracer may be
impaired i.e.- stunned myocardium (possibly related to loss of
the normal membrane potential or mitochrondrial
injury) [84,104].
A paradoxical perfusion pattern has also been described in
regions of myocardial necrosis with a patent coronary artery or
very good collateral flow to that segment [183]. A paradoxical
perfusion pattern can be found in up to 6.6% of perfusion scans
done on patients with prior MI [183]. The finding may be related
to non-transmural infarct [183]. Finally, shifting attenuation
artifact can aslo mimick the finding [217].
Imaging
in the early post-infarct period:
In the post-infarct period, patients with evidence of ischemia on myocardial perfusion imaging have a significantly higher risk for subsequent cardiac events compared to patients without ischemia [42,67]. The greater the extent of ischemia, the more likely patients are to have a subsequent event [42,50]. Gated LVEF determination also provides prognostic information. Patients with post-infarct LVEF's less than 40% have a 3 fold increased risk for cardiac death or infarction [42]. Even with submaximal exercise, SPECT imaging is more likely to demonstrate regions of ischemia compared to ECG findings alone [50]. Pharmacologic stress imaging can also be employed as early as one to two days following acute myocardial infarction allowing very early risk stratification [50]. With pharmacologic stress, patients with small (less than 10%) or predominantly nonischemic defects can generally be treated conservatively, while patients with more extensive ischemia should be managed more aggressively due to their higher risk for subsequent cardiac event [50].
The INSPIRE trial was performed to determine risk stratification and best treatment method based upon perfusion exam findings in the early post MI period [106]. Overall, the extent of the infarct and the amount of residual ischemia were the best predictors of subsequent events [106]. Patients with small perfusion defects (less than 20% LV) were considered low risk and were targeted to receive medical therapy [106]. The subsequent rate of re-infarction/death in this group was 1.8% and coronary revascularization did not improve outcome in this group [106]. The OAT trial has also demonstrated no prognostic advantage to stenting of an occluded infarct-related artery compared to medical therapy alone in patients with minimal or no residual ischemia [106]. Patients with large (over 20%) defects and little (under 10%) ischemia were felt to be intermediate risk, but thought unlikely to benefit from coronary revascularization [106]. The cardiac event rate in this group was higher, but there was no signficant difference between medical treatment versus revascularization (13.9% versus 14.7%) [106]. Patients with large (over 20%) defects and ischemia (greater than 10%) with LVEF's under 35% were defined as high risk and encouraged to undergo revascularization [106]. This group was found to benefit the most from revascularization with a decrease in cardiac events from 32% to 10% [106]. For this same group of patients (over 20% perfusion defect and more than 10% ischemia) but preserved LV function, intensive medical therapy was as effective as coronary revascularization [106].
Patients
with known or suspected CAD
Technetium agent myocardial perfusion imaging yields
incremental prognostic information towards the identification of
patients at risk for cardiac events [15]. Patients with adequate
exercise stress and a normal perfusion exam have an
overall annualized cardiac event (MI or cardiac death) rate of
less than 0.5-2% per year [8,10,15,41,44,186].
From
a
multicenter
registry, the annualized cardiac death rate for patients with a
normal tetrofosmin exam has also
been shown to be very low (0.6%) [40]. The event rate is very
low in patients with normal scans that have intact functional
capabilities with a cardiac death rate of 0.1% per year for
patients achieving >/= 10 mets on exercise stress testing
[187].
However, even if the perfusion scan is normal, it has been demonstrated that the cardiac death or non-fatal infarction rate is higher in patients with previously document coronary artery disease [44,150,186] (about 2% per year [49]). Other factors associated with a higher risk for cardiac events, despite a normal perfusion scan include: pharmacologic stress (about 2% annual cardiac death or non-fatal MI with normal scan [144]), diabetes mellitus (annual hard event rate of 2-3%) [145], males gender, coronary artery calcification (Agaston score >400) [187], underlying LV dysfunction (LVEF <45%) [187], extent of resting perfusion abnormalities (>10% of the myocardium) [187], and increasing age [44,49,52]. Thus, in specific patient populations, close follow-up may be warranted with serial testing in as early as one year [49]. Additional testing with coronary artery calcium scoring may also be beneficial in patients with intermediate clinical risk as the exam may detect preclinical CAD that would prompt risk factor modification [108]. [150].
Conversely, an abnormal perfusion exam which demonstrates a reversible defect is associated with a significantly increased risk for cardiac events [10,112]. An increased number of reversible defects, the presence of defects in multiple vascular territories, and the severity and extent of reversible defects are all associated with an increased risk for subsequent cardiac event [10,14,52]. The most severe reversible perfusion defect does not necessarily reflect the cardiac territory at greatest risk for subsequent infacrtion [12]. Severe stenoses are more likely to be associated with collateral circulation and infarction is more likely to complicate acute thrombosis of a mildly obstructive plaque [12]. In patients with prior MI, even the presence of fixed perfusion defects can indicate an increased risk for subsequent cardiac events- especially for patients with abnormalities in a multi-vessel distribution [62].
The results of the myocardial perfusion exam can also aid in risk stratification and provide a guide for the most effective treatment options [15]. Patients with mildly abnormal exams have a greater risk for myocardial infarction, rather than cardiac death, and this subgroup of patients would likely benefit most from initial medical treatment [15]. In patients with severely abnormal scans indicating extensive ischemia (over 10-20% of the myocardium), early revascularization will result in a decreased rate of cardiac death compared to a similar group of patients treated with medical therapy [15,118,174]. In patients with no or low amounts of inducible ischemia (less than 10% of the myocardium) medical therapy has been shown to be superior to revascularization [118]. Referral to catheterization and the accompanying costs may not be required if symptoms can be controlled with medical therapy [15]. Perfusion imaging can also be used to quantify the effectiveness of optimal medical therapy [174]. Patients not showing a reduction in their ischemic burden by at least 5% of the myocardium have worsening death or myocardial infarction rates compared with patients exhibiting greater reductions in ischemia [174]. Thus, a failure to reduce a patient's ischemic burden signifies high-risk status and intensification of OMT and consideration of repeat angiography for CAD progression and possible revascularization [174].
In general, patients with prior MI that are more likely to have
a cardiac event tend to be more symptomatic, are more frequently
diabetic, and are more likely to undergo adenosine stress
imaging [31]. However, cardiac event rates have also been shown
to increase as a function of SPECT abnormalities [31]. Very low
event rates of less than 1% are observed in patients with small
MI's and no or only mild ischemia [31]. Patients with moderate
or severe ischemia, and patients
with large MI's had a greater likelihood of having cardiac
events [31].
In diabetic patients:
CAD accounts for 70% of deaths among diabetic patients and diabetic have a 2-to-4 fold higher risk of cardiac events compared to non-diabetic patients [118]. Silent myocardial ischemia can be identified in 17-22% of asymptomatic diabetic patients, however, high risk scans (ischemia greater than 10% of the LV) are found in only 6% [118]. In contrast to the low annual event rate in patients with normal MPI exam (0.6%), asymptomatic diabetic subjects with normal or low-risk MPI have been shown to have an annual cardiac event rate (cardiac death and non-fatal MI) of 1.6% and an annual mortality rate of 3.6% [118]. Duration of diabetes and type of therapy also provide incremental prognostic information [158]. Patients with diabetes for 10 or more years and those that are on insulin therapy have been shown to be at greatest risk for adverse cardiac events [158].
In patients with chronic renal failure:
CRF markedly increases the risk of cardiac events and cardiovascular disease is the leading cause of death in these patients [161]. Even CRF patients with normal perfusion scans have been shown to have a higher annualized adverse event rate- up to 4.9% per year [231]. The presence of perfusion abnormalities on MPI have been found to predict adverse cardiac events in asymptomatic CRF patients [161].
In patients with peripheral vascular disease:
Peripheral atherosclerotic disease (PAD) is associated with a 20-60% increased risk of MI and a 2- to 6 fold increased risk for coronary heart disease mortality [161]. Ischemia can be found on MPI in 55% of patients with PAD, in 37% of patients with AAA, and in 73% of patients with both PAD and AAA (interestingly, only 17% reported a history of angina) [161].
In patients with stable angina pectoris:
Based upon Bayesian analysis, the probability of CAD does not change significantly in patients with a high pre-test probability of CAD and negative perfusion imaging results [77]. However- it is not known if these patients remain at high risk for subsequent cardiac events [77]. In a recent study [77] the annual event rate for patients with stable angina and a normal perfusion exam was 1.2%, compared to an event rate of 3.9% if the perfusion scan was abnormal [77]. Mutli-vessel perfusion abnormalities were associated with the greatest risk for subsequent cardiac events (4.8%) [77]. Both fixed and reversible perfusion abnormalities were predictive of mortality [77]. In this study, a policy of proceeding to angiography without performing a perfusion exam would have resulted in subjecting 30% of patients to an expensive and invasive procedure, despite an expected good outcome without intervention [77]. The study concluded that SPECT imaging provides independent information for predicting outcome in patients with stable angina [77].
In HIV:
Patients with HIV have accelerated coronary atherosclerosis [161].
Following angioplasty and stent placement in
patients without myocardial infarction and following coronary
artery bypass grafting:
More than 1 million coronary angioplasty and stent implantation
procedures are performed annually [79]. The COURAGE trial
concluded that percutaneous
coronary intervention (PCI) could be safely deferred in patients
with stable CAD- even those with extensive multivessel
disease and inducible eischemia, provided that omptimal medical therapy (OMT) was
instituted and maintained [113]. About 30% of the 1 million PVI
procedures performed annually are done on stable CAD patients-
hence 300,000 procedures could be deferred based upon the trial
results [113]. Classic teaching is that stress coronary flow
decreases significantly only when the narrowing exceeds 70%,
however, not all of these lesions may have compromised flow
reserve, and conversely, the fractional flow reserve (FFR) can
be abnormal in up to 35% of patients with stenoses between
50-70% [234]. The use of FFR- defined as the ratio of pressure
proximal and distal to a stenotic lesion during
vasodilator-induce hyperemia- may aid in determining the
physiologic significance of anatomic lesions [168]. A fractional
flow reserve of greater than 0.75 suggests that PCI can be
deferred without increased patient risk [168,235]. Conversely,
event rates increased when lesions with a fractional flow
reserve lower than 0.75 were not revascularized [168]. Other
authors suggest that patients with more than 10-15% ischemic
myocardium at MPI (by either SPECT or PET) should also be
referred for invasive assessment [168].
The incidence of re-stenosis following percutaneous coronary intervention (PCI) is and patients are not necessarily symptomatic [79]. SPECT imaging can aid in identifying patients at risk for a poor long term outcome [90]. Reversible perfusion defects can be found in 30-50% of patients following coronary balloon angioplasty and the finding is associated with a high risk of restensosis [28]. Restenosis is found in 52-75% of patients with reversible defects on early imaging (less than 2 months following PCI), compared to a 12-17% incidence in patients with normal scans [79]. The incidence of hard cardiac events is also higher in patients with inducible ischemic changes [90]. The overall sensitivity for detecting restenosis by SPECT ranges from 76-94% (mean 89%), and the specificity ranges from 46-84% (mean 75%) [90]. False positive exams may occur as a result of stunned vasa vasorum in the coronary artery affecting vasodilator reserve, and this can be minimized by delaying imaging at least 4 weeks following the procedure [90].
In spite of a good angiographic appearance, intravascular ultrasound has shown that some of the reversible perfusion abnormalities can be explained by an inadequate result [28]. Other etiologies which may explain reversible perfusion abnormalities in post-angioplasty patients include a transient defect in distal vessel autoregulation or a local spasm of the vessel wall at the site of dilatation [28].
Coronary stents: Following coronary stent placement, reversible perfusion abnormalities can be found in up to 10-17% of patients [28,124]. The reversible perfusion abnormalities may be related to developing restenosis, impairment of the microvascular response to stress, microvascular stunning due to particulate embolization associated with the procedure, unapparent angiograhpic obstruction at or adjacent to the stent (unapparent stent-edge dissection or new accumulations of focally extruded plaque) or unrecognized incomplete stent expansion [28]. However, the finding is also associated with an increased risk for restenosis and a higher cardiac event rate (up to 32% [124]) [28,124]. Up to 68% of patients with stent target vessel ischemia can be asymptomatic [124]. Drug-eluting stents reduce angiographic restenosis significantly [124]. Target vessel ischemia is found in about 5-7% of patients with drug eluting stents at 6 months following stent placement [124]. As with non-eluting stents, target vessel ischemia is also associated with a higher cardiac event rate [124].
Long-term adverse outcomes are increasing in the CABG population because multivessel surgery is common, requiring improved surveillance for graft patency, progression of plaque burden, and LV function [79]. In patients that have undergone revascularization with either percutaneous intervention or CABG, the presence of reversible perfusion abnormalities adds incremental prognostic information as this finding is associated with a significantly increased risk for subsequent cardiac events- even in asymptomatic patients [45,79]. A normal study in this same cohort, is associated with a very low risk for cardiac events [45].
Evaluation of Myocardial Viability
See Myocardial
Viability section
CZT camera cardiac imaging:
The dedicated cardiac CZT camera consists of 19 CZT solid state
detector modules positioned at different angles around the
patient that acquire the images simultaneously [222]. Each
detector is associated with a pinhole collimator that is focused
on a 19 cm quality field of view [222]. Acquisition times are
reduced by a factor of 2.5-7.5 compared to Anger cameras [222].
However, simultaneous acquisition means that a single occurence
of motion will affect ALL detectors, and due to the reduced
imaging time, a greater proportion of the overal
acquisition/count statistics [222]. Motion can be creep (a
gradual creep upward in position of the heart throughout
acquisition) or single/multiple occurences of returning or
non-returning motion [222].
REFERENCES:
(1) J Nucl Med 1994; Germano G, et al. A
quantitative phantom analysis of artifacts due to hepatic
activity in technetium-99m myocardial perfusion SPECT studies.
35: 356-59
(2) Am J Cardiology 1990; Maddahi J, et al. Myocardial perfusion imaging with technetium-99m sestamibi SPECT in the evaluation of coronary artery disease. 66: 55E-62E
(3) Eur J Nucl Med 1988; Taillefer et al. Myocardial perfusion imaging with 99mTc-methoxy-isobutyl-isonitrile (MIBI): comparison of short and long time intervals between rest and stress injections. Preliminary results. 13: 515-522
(4) Radiol Clin North Am 1993; Borges-Neto S, Coleman RE. Radionuclide ventricular function analysis.31: 817-830
(5) J Nucl Med 1994; Kiat H, et al. Comparative feasibility of separate or simultaneous rest thallium-201/stress technetium-99m-sestamibi dual-isotope myocardial perfusion SPECT. 35: 542-48
(6) J Nucl Med 1994; Sciagra R, et al. Evaluation of coronary artery disease using technetium-99m-sestamibi first-pass and perfusion imaging with dipyridamole infusion. 35: 1254-64
(7) J Nucl Med 1992; Gibson WS, et al. Serial tomographic imaging with technetium-99m-sestamibi for the assessment of infarct-related arterial patency following reperfusion therapy. 33: 2080-85
(8) J Nucl Med 1994; Brown KA, et al. Prognostic value of normal technetium-99m-sestamibi cardiac imaging. 35: 554-57
(9) J Am Coll Cardiol 1993; Varetto T, et al. Emergency room technetium-99m sestamibi imaging to rule out acute myocardial ischemic events in patients with nondiagnostic electrocardiograms. 22: 1804-8
(10) Chest 1999; Mandalapu BP, et al. Technetium Tc 99m Sestamibi myocardial perfusion imaging. Current role for evaluation of prognosis. 115: 1684-1694
(11) J Nucl Med 2000; Walicka MA, et al. Morphological transformation of C3H 10T1/2 cells by 99mTc-Cardiolite. 41: 1545-1551
(12) J Nucl Med 2001; Candell-Riera J, et al. Relationship between the location of the most severe myocardial perfusion defects, the most severe coronary artery stenosis, and the site of subsequent myocardial infarction. 42: 558-563
(13) J Nucl Med 1995; June; p.952-55
(14) J Nucl Med 2001; Sharir T, et al. Prediction of myocardial infarction versus cardiac death by gated myocardial perfusion SPECT: Risk stratification by the amount of stress-induced ischemia and the poststress ejection fraction. 42: 831-837
(15) Ciculation 1998; Hachamovitch R, et al. Incremental prognostic value of myocardial perfusion single photon emission computed tomography for the prediction of cardiac death. Differential stratification for risk of cardiac death and myocardial infarction. 97: 535-543
(16) J Nucl Med 1993; Villanueva-Meyer J, et al. Assessment of myocardial perfusion defect size after early and delayed SPECT imaging with technetium-99m-hexakis 2-methoxyisobutyl isonitrile after stress. 34: 187-192
(17) Eur J Nucl Med 1994; Klopper RS, et al. The excretion of radiopharmaceuticals in human breast milk: Additional data and dosimetry. 21: 144-153
(18) Semin Nucl Med 1995; Newhouse HK, Wexler JP. Myocardial perfusion imaging for evaluating interventions in coronary artery disease. 25: 15-27
(19) J Nucl Med 1995; Mahmood S, et al. Combined rest thallium-201/stress technetium-99m-tetrofosmin SPECT: feasibility and diagnostic accuracy of a 90-minute protocol. 36: 932-935
(20) J Nucl Med 1995; Cuocolo A, et al. Technetium-99m-tetrofosmin regional myocardial uptake at rest: relation to severity of coronary artery stenosis in previous myocardial infarction. 36: 907-13
(21) J Nucl Med 2001; Tanaka R, Nakamura T. Time course evaluation of myocardial perfusion after reperfusion therapy by 99mTc-tetrofosmin SPECT in patients with acute myocardial infarction. 42: 1351-1358
(22) J Nucl Med 2001; Taki J, et al. 99mTc-sestamibi retention characteristics during pharmacologic hyperemia in human myocardium: comparison with coronary flow reserve measured by doppler flowire. 42: 1457-1463
(23) J Nucl Med 2001; Nakajima K, et al. Accuracy of ventricular volume and ejection fraction measured by gated myocardial SPECT: Comparison of 4 software programs. 42: 1571-1578
(24) J Nucl Med 1997; Munch G, et al. Myocardial technetium-99m-tetrofosmin and technetium-99m-sestamibi kinetics in normal subjects and patients with coronary artery disease. 38: 428-432
(25) J Nucl Med 1999; Nichols K, et al. Influence of arrhythmias on gated SPECT myocardial perfusion and function quantification. 40: 924-934
(26) J Nucl Med 2001; Sharir T, et al. Quantitative analysis of regional motion and thickening by gated myocardial perfusion SPECT: Normal heterogeneity and criteria for abnormality. 42: 1630-1638
(27) J Am Coll CArdiol 1998; Heller GV, et al. Clinical value of acute rest technetium-99m tetrofosmin tomographic myocardial perfusion imaging in patients with acute chest pain and nondiagnostic electrocardiograms. 31: 1011-17
(28) J Nucl med 2001; Rodes-Cabau J, et al. Frequency and clinical significance of myocardial ischemia detected early after coronary stent placement.
(29) J Nucl Cardiol 2001; Choy JB, Leslie WD. Clinical correlates of Tc-99m sestamibi lung uptake. 8: 639-44
(30) J Nucl Med 2002; Sciagra R, et al. Comparison of dobutamine echocardiography and 99mTc-sestamibi tomography for prediction of left ventricular outcome after acute myocardial infarction treated with successful primary coronary angioplasty. 43: 8-14
(31) J Nucl Cardiol 2002; Zellweger MJ, et al. Risk stratification in patients with remote prior myocardial infarction using rest-stress myocardial perfusion SPECT: prognostic value and impact on referral to early catheterization. 9: 23-32
(32) J Nucl Med 2002; Shirai N, et al. Incremental value of assessment of regional wall motion for detection of multivessel coronary artery disease in exercise 201Tl gated myocardial perfusion imaging. 43: 443-450
(33) Am J Cardiol 2001; Kroll D, et al. Prognostic value of stress-gated Tc-99m sestamibi SPECT after acute myocardial infarction. 87: 381-86
(34) J Nucl Med 2002; Taki J, et al. Electrocardiographic gated 99mTc-MIBI SPECT for functional assessment of patients after coronary artery bypass surgery: comparison of wall thickening and wall motion analysis. 43: 589-595
(35) J Nucl Cardiol 2002; Knott JC, et al. Impact of acute chest pain Tc-99m sestamibi myocardial perfusion imaging on clinical management. 9: 257-262
(36) J Nucl Cardiol 2002; Simones MV, et al. Prediction of left ventricular wall motion recovery after acute myocardial infarction by Tl-201 gated SPECT: incremental value of integrated contractile reserve assessment. 9: 294-303
(37) J Nucl Med 2002; Feng B, et al. Calculation of the left ventricular ejection fraction without edge detection: application to small hearts. 43: 786-794
(38) J Nucl Cardiol 2002; Vallejo E, et al. Variability of serial same-day left ventricular ejection fraction using quantitative SPECT. 9: 377-84
(39) J Nucl Cardiol 2002; Paul AK, et al. Characteristics of regional myocardial stunning after exercise in gated myocardial SPECT. 9: 388-394
(40) J Nucl Med 2003; Shaw LJ, et al. Prognostic value of normal exercise and adenosine 99mTc-tetrofosmin SPECT imaging results from the multicenter registry of 4,728 patients. 44: 134-139
(41) J Nucl Cardiol 2003; Elhendy A, et al. Long-term prognosis after a normal exercise stress Tc-99m sestamibi SPECT study. 10: 261-266
(42) J Nucl Med 2003; Spinelli L, et al. Prognostic value of combined assessment of regional left ventricular function and myocardial perfusion by dopamine and rest gated SPECT in patients with uncomplicated acute myocardial infarction. 44: 1023-1029
(43) J Nucl Cardiol 2003; Verberne HJ, et al. Stress-related variations in left ventricular function as assessed with gated myocardial perfusion SPECT. 10: 456-463
(44) J Nucl Cardiol 2003; Beller GA. Clinical value of myocardial perfusion imaging in coronary artery disease. 10: 529-542
(45) J Nucl Cardiol 2003; Elhendy A, et al. Risk stratification of patients after myocardial revascularization by stress Tc-99m tetrofosmin myocardial perfusion tomography. 10: 615-622
(46) J Nucl Cardiol 2003; Kasai T, et al. Impact of gating errors with electrocardiography gated myocardial perfusion SPECT. 10: 709-711
(47) J Nucl Cardiol 2004; Beller GA, Bergmann SR. Myocardial perfusion imaging agents: SPECT and PET. 11: 71-86
(48) J Nucl Cardiol 2004; Allman KC, Freedman SB. Emergency department assessment of patients with acute chest pain: myocardial perfusion imaging, blood tests, or both? 11: 87-89
(49) J Nucl Cardiol 2004; Shaw LJ, Iskandrian AE. Prognostic value of gated myocardial perfusion SPECT. 11: 171-185
(50) J Nucl Cardiol 2004; Mahmarian JJ, et al. Role of nuclear cardiac imaging in myocardial infarction: postinfarction risk stratification. 11: 186-209
(51) J Nucl Med 2004; Go V, et al. The diagnostic and prognostic value of ECG-gated SPECT myocardial perfusion imaging. 45: 912-921
(52) J Nucl Cardiol 2004; Travin MI, et al. The prognostic value of ECG-gated SPECT imaging in patients undergoing stress Tc-99m sestamibi myocardial perfusion imaging. 11: 253-262
(53) J Nucl Cardiol 2004; Grossman G, et al. Quantitative Tc-99m sestamibi attenuation-corrected SPECT: development and multicenter trial validation of myocardial perfusion stress gender-independent normal database in an obese population. 11: 263-272
(54) J Nucl Cardiol 2004; Heller GV, et al. Clinical value of attenuation correction in stress-only Tc-99m sestamibi SPECT imaging. 11: 273-81
(55) Radiology 2004; Borger-Neto S, et al. Outcome prediction in patients at high risk for coronary artery disease: comparison between 99mTc tetrofosmin and 99mTc setamibi. 232: 58-65
(56) J Nucl Cardiol 2004; Kontos MC, Wackers FJ. Acute rest myocardial perfusion imaging for chest pain. 11: 470-481
(57) J Nucl Med 2004; Meine TJ, et al. The additive value of combined assessment of myocardial perfusion and ventricular function studies. 45: 1721-1724
(58) J Nucl Cardiol 2004; Druz RS, et al. Postischemic stunning after adenosine vasodilator stress. 11: 534-541
(59) J Nucl Med 2004; Kasama S, et al. Dobutamine stress 99mTc-tetrofosmin quantitative gated SPECT predicts improvement of cardiac function after Carvedilol treatment in patients with dilated cardiomyopathy. 45: 1878-1884
(60) J Nucl Cardiol 2004; Lipiecki J, et al. Influence of infarct-zone viability detected by rest Tc-99m sestamibi gated SPECT on left ventricular remodeling after acute myocardial infarction treated by percutaneous transluminal coronary angioplasty in the acute phase. 11: 673-81
(61) J Nucl Cardiol 2004; Toba M, et al. Usefulness of gated myocardial perfusion SPECT imaging soon after exercise to identify postexercise stunning in patients with single-vessel coronary artery disease. 11: 697-703
(62) J Nucl Cardiol 2004; Elhendy A, et al. Prognostic value of stress Tc-99m tetrofosmin SPECT in patients with previous myocardial infarction: impact of scintigraphic extent of coronary artery disease. 11: 704-709
(63) J Nucl Med 2004; Abidov A, et al. Integration of automatically measured transient ischemic dilatation ratio into interpretation of adenosine stress myocardial perfusion SPECT for detection of severe and extensive CAD. 45: 1999-2007
(64) J Nucl Med 2005; Leslie WD, et al. Prognostic value of automated quantification of 99mTc-sestamibi myocardial perfusion imaging. 46: 204-211
(65) J Nucl Cardiol 2005; Petix NR, et al. Can the reversible regional wall motion abnormalities on stress gated Tc99m sestamibi SPEC predict a future cardiac event? 12: 20-31
(66) J Nucl Cardiol 2005; Djaballah W, et al. Gated SPECT assessment of left ventricular function is sensitive to small patient motions and to low rates of triggering errors: a comparison with equilibrium radionuclide angiography. 12: 78-85
(67) J Nucl Med 2005; Acampa W, et al. Prognostic value of myocardial ischemia in patients with uncomplicated acute myocardial infarction: direct comparison of stress echocardiography and myocardial perfusion imaging. 46: 417-423
(68) J Nucl Cardiol 2005; Thompson RC, et al. Value of attenuation correction on ECG-gated SPECT myocardial perfusion imaging related to body mass index. 12: 195-202
(69) J Nucl Med 2005; Fricke E, et al. Attenuation correction of myocardial SPECT perfusion images with low-dose CT: evaluation of the method by comparison with perfusion PET. 46: 736-744
(70) J Nucl Card 2005; Abidov A, Berman DS. Transient ischemic dilatation associate with poststress myocardial stunning of the left ventricle in vasodilator stress myocardial perfusion SPECT: true marker of severe ischemia? 12: 258-260
(71) J Nucl Med 2005; Schaefer WM, et al. Quantification of left ventricular volumes and ejection fraction from gated 99mTc-MIBI SPECT: MRI validation and comparison of Emory cardiac tool box with QGS and 4D-MSPECT. 46: 1256-1263
(72) J Nucl Med 2005; Emmett L, et al. The role of left ventricular hypertrophy and diabetes in the presence of transient ischemic dilatation of the left ventricle on myocardial perfusion SPECT images. 46: 1596-1601
(73) Radiology 2005; Utsunomiya D, et al. Object-specific attenuaiton correction at SPECT/CT in thorax: optimization of respiratory protocol for image registration. 237: 662-669
(74) J Nucl Cardiol 2005; De Winter O, et al. Incremental prognostic value of combined perfusion and function assessment during myocardial gated SPECT in patients aged 75 years or older. 12: 662-670
(75) J Nucl Cardiol 2005; Masood Y, et al. Clinical validation of SPECT attenuation-correction using x-ray computed tomography-derived attenuation maps: multicenter clinical trial with angiographic correlation. 12: 676-686
(76) J Nucl Med 2005; Heston TF, Sigg DM. Quantifying transient ischemic dilatation using gated SPECT. 46: 1990-1996
(77) J Nucl Med 2005; Elhendy A, et al. Risk stratification of patient with angina pectoris by stress 99mTc-tetrofosmin myocardial perfusion imaging. 46: 2003-2008
(78) J Nucl Cardiol 2006; Thompson RC, Cullom SJ. Issues regarding radiation dosage of cardiac nuclear and radiography procedures. 13: 19-23
(79) J Nucl Cardiol 2006; Adams GL, et al. Role of nuclear imaging after coronary revascularization. 13: 163-169
(80) J Nucl Cardiol 2004; Druz RS, et al. Postischemic stunning after adenosine vasodilator stress. 11: 534-541
(81) J Nucl Cardiol 2006; Hung GU, et al. Worsening of left ventricular ejection fraction induced by dipyridamole on Tl-201 gated myocardial perfusion imaging predicts significant coronary artery disease. 13: 225-232
(82) J Nucl Cardiol 2006; Abidov A, et al. Gated SPECT in assessment of regional and global left ventricular function: major tool of modern nuclear imaging. 13: 261-279
(83) J Nucl Cardiol 2006; Rivero A, et al. Attenuation correction reveals gender-related differences in the normal values of transient ischemic dilation index in rest-exercise stress sestamibi myocardial perfusion imaging. 13: 338-344
(84) J Nucl Cardiol 2006; Sugiura T, et al. Usefulness of Tc-99m methoxyisobutylisonitrile scintigraphy for evaluating congestive heart failure. 13: 64-68
(85) J Nucl Cardiol 2006; Boger LA, et al. Best patient preparation before and during radionuclide myocardial perfusion imaging studies. 13: 98-110
(86) J Nucl Cardiol 2006; Pham R, Bellezuoli E. Diffuse pulmonary uptake of Tc-99m sestamibi due to chemical pneumonitis. 13: 127-129
(87) J Nucl Med 2006; Goetze S, et al. Clinically significant abnormal findings on the "nondiagnostic" CT portion of low-amperage-CT attenuation-corrected myocardial perfusion SPECT/CT studies. 47: 1312-1318
(88) J Nucl Cardiol 2006; Akinboboye O, et al. Technetium tetrofosmin myocardial perfusion imaging in women. 13: 603-604
(89) J Nucl Cardiol 2006; Elhendy A, et al. Accuracy of stress Tc-99m tetrofosmin myocardial perfusion tomography for the diagnosis and localization of coronary artery disease in women. 13: 629-634
(90) J Nucl Cardiol 2006; Shaw LJ, et al. Gated myocardial perfusion single photon emission computed tomography in the clinical outcomes utilizing revascularization and aggressive drug evaluation (COURAGE) trial, veterans administration cooperative study no. 424. 13: 685-698
(91) J Nucl Cardiol 2006; Higgins JP. Increased right ventricular uptake on stress SPECT myocardial perfusion images in a patient with severe coronary artery disease. 13: 725-727
(92) J Nucl Cardiol 2006; Wyrick JJ, Wei K. Cardiac imaging in the evaluation of patients presenting to the emergency department with chest pain. 13: 749-755
(93) J Nucl Cardiol 2006; Hachamovitch R, et al. Predicting therapeutic benefit from myocardial revascularization procedures: are measurements of both resting left ventricular ejection fraction and stress-induced myocardial ischemia necessary? 13: 768-778
(94) J Nucl Cardiol 2006; Travin MI. Revascularize only for ischemia, especially if left ventricular function is poor. 13: 742-746
(95) J Nucl Cardiol 2006; Verberne HJ, et al. Multicenter intercomparison assessment of consistency of left ventricular function from a gated cardiac phantom. 13: 801-810
(96) J Nucl Cardiol 2006; Ibrahim DY, et al. Optimal SPECT processing and display: making bad studies look good to get the right answer. 13: 855-866
(97) J Nucl Med 2004; Fricke H, et al. A method to remove artifacts in attenuation-corrected myocardial perfusion SPECT introduced by misalignment between emission scan and CT-derived attenuation maps. 45: 1619-1625
(98) J Nucl Cardiol 2007; Garcia EV. SPECT attenuation correction: an essential tool to realize nuclear cardiology's manifest destiny. 14: 16-24
(99) J Nucl Cardiol 2007; Dorbala S, et al. Prognostic value of SPECT myocardial perfusion imaging in patients with elevated cardiac troponin I levels and atypical clinical presentation. 14: 53-58
(100) J Nucl Cardiol 2007; Hida S, et al. Diagnostic value of left ventricular function after stress and at rest in the detection of multivessel coronary artery disease as assessed by electrocardiogram-gated SPECT. 14: 68-74
(101) Nucl Cardiol 2007; Singh B, et al. Attenuation artifact, attenuation correction, and the future of myocardial perfusion SPECT. 14: 153-164
(102) J Nucl Cardiol 2007; Burque JM, et al. Mortality risk associated with ejection fraction differs across resting nuclear perfusion findings. 14: 165-173
(103) J Nucl Cardiol 2007; Goetze S, Wahl RL. Prevalence of misregistration between SPECT and CT for attenuation-corrected myocardial perfusion SPECT. 14: 200-206
(104) J Nucl Cardiol 2007; Matsuo S, et al. A novel clinical indicator using Tc-99m sestamibi for evaluating cardiac mitochondrial function in patients with cardiomyopathies. 14: 215-20
(105) J Nucl Cardiol 2007; Mujtaba B, et al. Anaphylactic reaction to Tc-99m sestamibi (Cardiolite) during pharmacologic myocardial perfusion imaging. 14: 256-258
(106) J Nucl Cardiol 2007; Mahmarian JJ, et al. Risk statification after acute myocardial infarction: is it time to reassess? Implications from the INSPIRE trial. 14: 282-292
(107) J Nucl Cardiol 2007; Sciagra R, et al. Influence of the postexercise acquisition delay on the detection of functional abnormalities in sestamibi-gated SPECT. 14: 334-340
(108) J Nucl Med 2007; Schepis T, et al. Added value of coronary artery calcium score as an adjunct to gated SPECT for the evaluation of coronary artery disease in an intermediate-risk population. 48: 1424-1430
(109) J Nucl Cardiol 2007; Germano G, et al. Quantitation in gated perfusion SPECT imaging: the Cedars-Siani approach. 14: 433-454
(110) J Nucl Cardiol 2007; Smelley MP, et al. A hypertensive response to exercise is associated with transient ischemic dilatation on myocardial perfusion SPECT. 12: 537-543
(111) J Nuc Med 2007; Giorgetti A, et al. Feasibility and diagnostic accuracy of a gated SPECT early-imaging protocol: a multicenter study of the myoview imaging optimization group. 48: 1670-1675
(112) J Nucl Cardiol 2007; Kapetanopoulos A, et al. Regional wall-motion abnormalities on post-stress ekectrocardiographic-gated technetium-99m sestamibi single-photon emission computed tomography imaging predict cardiac events. 14: 810-817
(113) J Nucl Cardiol 2008; Beller GA. What can be learned from the COURAGE trial. 15: 1-2
(114) J Nucl Med 2006; Thompson K, et al. Is septal glucose metabolism altered in patients with left bundle branch block and ischemic cardiomyopathy. 47: 1763-1768
(115) J Nucl Cardiol 2007; D'Orio Nishioka SA, et al. Cardiac sympathetic activity pre and post resynchronization therapy evaluated by 123I-MIBG myocardial scinitgraphy. 14: 852-859
(116) J Nucl Med 2007; Henneman MM, et al. Nuclear imaging in cardiac resynchronization therapy. 48: 2001-2010
(117) J Nucl Cardiol 2008; Port SC. Timing is everything. 15: 10-12
(118) J Nucl Cardiol 2008; Yerramasu A, et al. Cardiac computed tomography and myocardial perfusion imaging for risk stratification in asymptomatic diabetic patients: a critical review. 15: 13-22
(119) J Nucl Cardiol 2008; Tadehara F, et al. Feasibility of a rapid protocol of 1-day single-isotope rest/adenosine stress Tc-99m sestamibi ECG-gated myocardial perfusion imaging. 15: 35-41
(120) J Nucl Cardiol 2008; Harel F, et al. Comparison of left ventricular contraction homogeneity index using SPECT gated blood pool imaging and planar phase analysis. 15: 80-85
(121) J Nucl Cardiol 2008; Chen J, et al Assessment of left ventricular mechanical dyssynchrony by phase analysis of ECG-gated SPECT myocardial perfusion imaging. 15: 127-136
(122) J Nucl Med 2007; Henneman MM, et al. Can LV dyssynchrony as assessed with phase analysis on gated myocardial perfusion SPECT predict response to CRT? 48: 1104-1111
(123) Radiology 2008; Earls JP, et al. Prospectively gated transverse coronary CT angiography versus retrospectively gated helical technique: improved image quality and reduced radiation dose. 246: 742-753
(124) J Nucl Med 2008; Zellweger MJ, et al. Value and limitations of target-vessel ischemia in predicting late clinical events after drug-eluting stent implantation. 49: 550-556
(125) J Nucl Med 2008; Patel D, et al. Diastolic filling parameters derived from myocardial perfusion imaging can predict left ventricular end-diastolic pressure at subsequent cardiac catheterization. 49: 746-751
(126) J Nucl Med 2008; Wolak A, et al. Quantitative diagnostic performance of myocardial perfusion SPECT with attenuation correction in women. 49: 915-922
(127) J Nucl Med 2008; Sciagra R, et al. Assessment of the influence of atrial fibrillation on gated SPECT perfusion data by comparison with simultaneously acquired nongated SPECT data. 49: 1283-1287
(128) J Nucl Cardiol 2008; Emmett L, et al. Prospective evaluation of the impact of diabetes and left ventricular hypertrophy on the relationship between ischemia and transient ischemic dilatation of the left ventricle on single-day adenosine Tc-99m myocardial perfusion imaging. 15: 638-643
(129) J Nucl Cardiol 2008; Trimble MA, et al. Evaluation of mechanical dyssynchrony and myocardial perfusion using phase analysis of gated SPECT imaging in patients with left ventricular dysfunction. 15: 663-670
(130) J Nucl Med 2008; Van Kriekinge SD, et al. Automatic global and regional phase analysis from gated myocardial perfusion SPECT imaging: application to the characterization of ventricular contraction in patients with left bundle branch block. 49: 1790-1797
(131) J Nucl Cardiol 2008; Travin MI. The oft neglected rest study. 15: 739-742
(132) New Engl J Med 1993; Zaret BL, Wackers FJ. Nuclear cardiology (1). 329: 775-783
(133) J Nucl Cardiol 2001; Kaul S. The role of capillaries in determining coronary blood flow reserve: implications for stress-induced reversible perfusion defects. 8: 69
(134) J Nucl Med 2008; Vesely MR, Dilsizian V. Nuclear cardiac stress testing in the era of molecular medicine. 49: 399-413
(135) J Nucl Cardiol 2003; De Lorenzo A, et al. Use of atropine in patients with submaximal heart rate during exercise myocardial perfusion SPECT. 10: 51-55
(136) J Nucl Cardiol 2008; Shaw LJ, et al. Prognostic estimation of coronary artery disease risk with resting perfusion abnormalities and stress ischemia on myocardial perfusion SPECT. 15: 762-773
(137) J Nucl Cardiol 2009; Desai S, et al. Detection of multivessel coronary artery disease: looking beyind the extent of perfusion abnormalities. 16: 4-5
(138) J Nucl Cardiol 2009; Hida S, et al. Diagnostic value of left ventricular function after adenosine triphosphate loading and at rest in the detection of multi-vessel coronary artery disease using myocardial perfusion imaging. 16: 20-27
(139) J Nucl Cardiol 2009; Evangelista L, et al. Incremental prognostic value of cardiac single-photon emission computed tomography after nitrate administration in patients with ischemic left ventricular dysfunction. 16: 38-44
(140) J Nucl Cardiol 2009; Ohte N, et al. Impaired myocardial oxidative metabolism in the remote normal region in patients in the chronic phase of myocardial infarction and left ventricular remodelling. 16: 73-81
(141) J Nucl Med 2009; Gimelli A, et al. Stress/rest myocardial perfusion abnormalities by gated SPECT: still the best predictor of cardiac events in stable ischemic heart disease. 50: 546-553
(142) J Nucl Cardiol 2009; Saraste A, et al. Nuclear cardiology needs new "blood". 16: 180-183
(143) J Nulc Med 2009; Boogers MM, et al. Quantitative gated SPECT-derived phase analysis of gated myocardial perfusion SPECT detects left ventricular dyssynchrony and predicts response to cardiac resynchronization therapy. 50: 718-725
(144) J Nucl Med 2009; Shaw LJ, Narula J. Risk assessment and predictive value of coronary artery disease testing. 50: 1296-1306
(145) J Nucl Cardiol 2009; Miller TD, et al. Risk stratification in diabetic patients: a continuing challenge. 16: 486-489
(146) J Nucl Cardiol 2009; Prognostic value of myocardial perfusion scintigraphy in type 2 diabetic patients with mild, stable angina pectoris. 16: 524-532
(147) J Nucl Med 2009; Kennedy JA, et al. Directions and magnitudes of misregistration of CT attenuation-corrected myocardial perfusion studies: incidence, impact on image quality, and guidance for reregistration. 50: 1471-1478
(148) J Nucl Cardiol 2009; Atchley AE, et al. Use of phase analysis of gated SPECT perfusion imaging to quantify dyssynchrony in patients with mild-to-moderate left ventricular dysfunction. 16: 888-894
(149) J Nucl Cardiol 2009; Munoz del Romeral L, et al. The variable functional effects of the pacing site in normal and scarred ventricles. 16: 904-913
(150) J Nucl Cardiol 2010; Choudhary G, et al. The role of calcium score and CT angiography in the medical management of patients with normal myocardial perfusion imaging. 17: 45-51
(151) J Nucl Cardiol 2010; Gadiraju R, et al. Sustained supraventricular tachycardia resulting in a pattern of apparent "reversible transient ischemic dilatation" and an underestimated ejection fraction. 17: 153-157
(152) J Nucl Cardiol 2010; van der Veen BJ, et al. Transient ischemic dilatation ratio derived from myocardial perfusion scintigraphy: what are we looking at? 17: 207-215
(153) J Nucl Cardiol 2010; Giorgetti A, et al. Myocardial imaging with 99mTc-tetrofosmin: influence of post-stress acquisition time, regional radiotracer uptake, and wall motion abnormalities on the clinical result. 17: 276-285
(154) J Nucl Cardiol 2010; Boogers MJ, et al. Should mechanical dyssynchrony be assessed in patients with implantable cardioverter-defibrillators? 17: 354-358
(155) J Nucl Cardiol 2010; Harel F, et al. Gated blood-pool SPECT versus cardiac magnetic resonance imaging for the assessment of left ventricular volumes and ejection fraction. 17: 427-434
(156) J Nucl Cardiol 2010; Mahmarian JJ. Stress only myocardial perfusion imaging: is it time for a change? 17: 529-535
(157) J Nucl Cardiol 2010; Lin X, et al. Repeatability of left ventricular dyssynchrony and function parameters in serial gated myocardial perfusion SPECT studies. 17: 811-816
(158) J Nucl Cardiol 2011; Barmpouletos D, et al. Duration and type of therapy for diabetes: impact on cardiac risk stratification with stress electrocardiographic-gated SPECT myocardial perfusion imaging. 17: 1041-1049
(159) J Nucl Cardiol 2011; Isobe S, et al. Relation of 99mTc-sestamibi washout with myocardial properties in patients with hypertrophic cardiomyopathy. 17: 1082-1090
(160) J Nucl Med 2011; Uebleis C, et al. Electrocardiogram-gated 18F-FDG PET/CT hybrid imaging in patients with unsatisfactory response to cardiac resynchronization therapy: initial clinical results. 52: 67-71
(161) J Nucl Cardiol 2011; Hendel RC. et al. The role of radionuclide myocardial perfusion imaging for asymptomatic individuals. 18: 3-15
(162) J Nucl Cardiol 2011; Bilchick KC. Single photon emission computed tomography (SPECT) techniques for resynchronization: phase analysis and equilibrium radionuclide angiography. 18: 16-20
(163) J Nucl Cardiol 2011; Philippe L, et al. Tetrofosmin early time gated post-stress single-photon emission computed tomography imaging: feasibility and potential benefits. 18: 62-72
(164) J Nucl CArdiol 2011; Supariwala A, et al. Synergistic effect of coronary artery disease risk fractors on long-term survival in patients with normal exercise SPECT studies. 18: 207-214
(165) J Nucl Cardiol 2011; Valdiviezo C, et al. The significance of transient ischemic dilatation in the setting of otherwise normal SPECT radionuclide myocardial perfusion images. 18: 220-229
(166) J Nucl Cardiol
2011; Kontos MC. Myocardial
perfusion imaging in the acute care setting: does it still have
a role? 18: 342-350
(167) J Nucl Cardiol 2011; Fazel R, et al. Strategies for
defining an optimal risk-benefit ratio for stress myocardial
perfusion SPECT. 18: 385-392
(168) J Nucl Med 2011; Tamarappoo B, Hachamovitch R. Myocardial
perfusion imaging versus CT coronary angiography: when to use
which? 52: 1079-1086
(169) J Nucl Cardiol 2011; Fazel R, Shaw LJ. Radiation exposure
from radionuclide myocardial perfusion imaging: concerns and
solutions. 18: 562-565
(170) J Nucl Cardiol 2011; Williams KA, Ballapuram K. Radiation
exposure in diagnostic imaging- use, misuse, or abuse? Part I:
the background and science of medical radiation. 18: 566-569
(171) J Nucl Cardiol 2011; Henzlova MJ, Duvall WL. The future
of SPECT MPI: time and dose reduction. 18: 580-587
(172) J Nucl Cardiol 2011; Chen J, et al. SPECT myocardial
perfusion imaging for the assessment of left ventricular
mechanical dyssynchrony. 18: 685-694
(173) J Nucl Cardiol 2011; Mandour MA, et al. The prevalence
and predictive accuracy of quantitatively defined transient
ischemic dilatation of the left ventricle on otherwise normal
SPECT myocardial perfusion imaging studies. 18: 1036-1043
(174) J Nucl Med 2012; Xu YZ, et al. Impact of myocardial
scarring on outcomes of cardiac resynchronization therapy:
extent or location. 53: 47-54
(175) J Nucl Cardiol 2012; Bateman TM. Advantages and
disadvantages of PET and SPECT in a busy clinical practice. 19:
S3-11
(176) J Nucl Cardiol 2012; Verna E, et al. Evaluation of
baseline contractile reserve vs dyssynchrony as a predictor of
functional improvement and long term outcome after
resynchronization pacing therapy: a radionuclide stress study.
19: 53-62
(177) J Nucl Cardiol 2012; Al Jaroudi W, et al. Effect of
tracer dose on left ventricular mechanical dyssynchrony indices
by phase analysis of gated single photon emission computed
tomography myocardial perfusion imaging. 19: 63-72
(178) J Nucl Cardiol 2012; DePuey EG, et al. Patient-centered
imaging. 19: 185-215
(179) J Nucl Cardiol 2012; Nabi F, et al. Assessing risk in
acute chest pain: the value of stress myocardial perfusion
imaging in patients admitted through the emergency department.
19: 233-243
(180) J Nucl Cardiol 2012; Slomka P, et al. Quantitative
analysis of perfusion studies: strengths and pitfalls. 19:
338-346
(181) J Nucl Cardiol 2012; Port S. Cardiac dyssynchrony: we
have the tools. It is time to use them. 19: 420-423
(182) J Nucl Cardiol 2012; Xu Y, et al. Transient ischemic
dilatation for coronary artery disease in quantitative analysis
of same-day sestamibi myocardial perfusion SPECT. 19: 465-473
(183) J Nucl Cardiol 2012; Nazarena M, et al. Paradoxical
scintigraphic pattern in regions with myocardial necrosis on
myocardial perfusion gated SPECT with 99mTc-tetrofosmin.
19: 515-523
(184) J Nucl Cardiol 2012; Wells RG, et al. Comparing slow-
versus high speed CT for attenuation corretion of cardiac SPECT
perfusion studies. 19: 719-726
(185) J Nucl Cardiol 2012; Emmett L, et al. Comparative
assessment of rest and post-stress left ventricular volumes and
left ventricular ejection fraction on gated myocardial perfusion
imaging (MPI) and echocardiography in patients with transient
ischemic dilatation on adenosine MPI: myocardial stunning or
subendocardial hypoperfusion? 19: 735-742
(186) J Nucl Cardiol 2012; Schinkel AFL, et al. 15-year outcome
after normal exercise 99mTc-sestamibi myocardial
perfusion imaging: what is the duration of low risk after a
normal scan. 19: 901-906
(187) J Nucl Cardiol 2012; Shaw LJ, et al. Prognosis in the era
of comparative effectiveness research: where is nuclear
cardiology now and where should it be? 19: 1026-1043
(188) J Nucl Med 2012; Ludwig DR, et al. On the importance of
image gating for the assay of left ventricular mechanical
dyssynchrony using SPECT. 53: 1892-1896
(189) J Nucl Cardiol 2012; mAnanthasubramaniam K, Bhatti S.
Stress first myocardial perfusion imaging: is it time to put to
rest the "rest first" stratgey for most patients? 19: 1106-1109
(190) J Nucl Cardiol 2012; Duvall WL, et al. A model for the
prediction of a successful stress-first Tc-99m SPECT MPI. 19:
1124-1134
(191) J Nucl Cardiol 2012; Aljaroudi W, et al. Paradoxical
septal motion from prior coronary artery bypass graft surgery
does not impact left ventricular dyssynchrony by gated
myocardial perfusion imaging. 19: 1190-1197
(192) J Nucl Cardiol 2013; Henzlova MJ, et al. Stress-only
imaging: faster, cheaper, less radiation. So what's the hold up?
20: 17-19
(193) J Nucl Cardiol 2013; Mathur S, et al. Clinical value of
stress-only Tc-99m SPECT imaging: importance of attenuation
correction. 20: 27-37
(194) J Nucl Cardiol 2013; Petretta M, et al. Transient
ischemic dilation in SPECT myocardial perfusion imaging for
prediction of severe coronary artery disease in diabetic
patients. 20: 45-52
(195) J Nucl Cardiol 2013; Iskandar A, et al. Gender
differences in the diagnostic accuracy of SPECT myocardial
perfusion imaging: a bivariate meta-analysis. 20: 53-63
(196) J Nucl Cardiol 2013; Zafrir N, et al. Feasibility of
myocardial perfusion imaging with half the radiation dose in
obese patients using ordered-subset expectation maximization
with resolution recovery software. 20: 111-119
(197) Radiology 2013; Coelho-Filho OR, et al. MR myocardial
perfusion imaging. 266: 701-715
(198) J Nucl Cardiol 2013; Uretsky S, et al. Long-term outcomes
following a normal stress myocardial perfusion scan. 20: 715-718
(199) J Nucl Cardiol 2013; Ottenhof MJM, et al. 12-year outcome
after normal myocardial perfusion SPECT in patients with known
coronary artery disease. 20: 748-754
(200) J Nucl Cardiol 2013; Doukky R, et al. The prognostic
value of transient ischemic dilatation with otherwise normal
SPECT myocardial perfusion imaging: a cautionary note in
patients with diabetes and coronary artery disease. 20: 774-784
(201) J Nucl Cardiol 2013; Hakeem A, et al. Does hybrid imaging
have a role in cardiac risk evaluaiton of the pre-renal
transplant patient? 20: 963-965
(202) J Nucl Cardiol 2013; Zizek D, et al. Impact of myocardial
viability assessed by myocardial perfusion imaging on
ventricular tachyarrhythmias in cardiac resynchronization
therpay. 20: 1049-1059
(203) J Nucl Cardiol 2013; Abidov A, et al. Gated SPECT in
assessment of regional and global left ventricular function: an
update. 20: 1118-1143
(204) J Nucl Cardiol 2013; Gulati V, et al. The role of
radionuclide imaging in heart failure. 20: 1173-1183
(205) J Nucl Cardiol 2014; Acampa W, et al. Warranty period of
normal stress myocardial perfusion inmaging in diabetic
patients: a propensity score analysis. 21: 50-56
(206) J Nucl Cardiol 2014; Goldberg AS, et al. Prognostic value
of left ventricular mechanical dyssynchrony by phase analysis in
patients with non-ischemic cardiomyopathy with ejection fraction
35-50% and QRS < 150 ms. 21: 57-66
(207) J Nucl Cardiol 2014; Hage F. Left ventricular mechanical
dyssynchrony by phase analysis as a prognostic indicator in
heart failure. 21: 67-70
(208) J NUcl Cardiol 2014; Rozanski A, et al. Long-term
mortality following normal exercise myocardial perfusion SPECT
according to coronary disease risk factors. 21: 341-350
(209) J Nucl Med 2014; Halligan WT, et al. Transient ischemic
dilatation of the left ventricle on SPECT: correlation with
findings at coronary CT angiography. 55: 917-922
(210) J Nucl Cardiol 2014; Zafir N, et al. Prognostic value of
left ventricular dyssynchrony by myocardial perfusion-gated
SPECT in patients with normal and abnormal left ventriucular
functions. 21: 532-540
(211) J Nucl Cardiol 2014; Hage FG, Garcia EV. The independent
prognostic value of left ventricular dyssynchony. 21: 541-543
(212) J Nucl Cardiol 2014; Acampa W, et al. Prognostic value of
normal stress myocardial perfusion imaging in diabetic patients:
a meta-analysis. 21: 893-902
(213) J Nucl Cardiol 2014; Huang WS, et al. Relation of early
post-stress left ventricular dyssynchrony and the extent of
angiographic coronary artery disease. 21: 1048-1056
(214) J Nucl Cardiol 2014; Marsan NA, Bax JJ. The potential
role of gated myocardial perfusion SPECT imaging in patient
selection for cardiac resynchonization therapy. 21: 1072-1074
(215) J Nucl Cardiol 2014; Mut F, et al. Detection of
post-exercise stunning by early gated SPECT myocardial perfusion
imaging: results from the IAEA multi-center study. 21: 1168-1176
(216) J Nucl Cardiol 2015; Hage FG, et al. Review of
cardiovascular imaging in The Journal of Nuclear Cardiology in
2014: Part 3 of 2: myocardial perfusion imaging. 22: 714-719
(217) J Nucl Cardiol 2015; Pucar D, et al. "Reverse
redistribution" pattern on SPECT myocardial perfusion imaging
from endothelial dysfunction at rest and from subendocardial
ischemia. 22: 845-848
(218) J Nucl Cardiol 2015; Zellweger MJ. Looking at the whole
picture. 22: 901-902
(219) J Nucl Med 2015; Kitkungvan D, et al. Clinical utility of
enhanced relative activity recovery on systolic myocardial
perfusion SPECT: lessons from PET. 56: 1882-1888
(220) J Nucl Cardiol 2016; Reynolds SN, Kikut J. Adherence of
Tc-99m sestamibi to plastic syringes could complicate efforts in
dose reduction in MPI SPECT. 23: 256-264
(221) J Nucl Cardiol 2016; Bajaj NS, et al. The prognostic
value of non-perfusion variables obtained during vasodilator
stress myocardial perfusion imaging. 23: 390-413
(222) J Nucl Cardiol 2016; Redgate S, et al. A study to
quantify the effect of patient motion and develop methods to
detect and correct for motion during myocardial perfusion
imaging on a CZT solid-state dedicated cardiac camera. 23:
514-526
(223) J Nucl Cardiol 2016; Henzlova M, et al. ASNC imaging
guidelines for SPECT nuclear cardiology procedures: stress,
protocols, and tracers. 23: 606-639
(224) J Nucl Cardiol 2016; Soman P and Einstein AJ. Biologic
effects of radiation from cardiac imaging: new insights from
proteomic and genomic analyses. 23: 754-757
(225) J Nucl Cardiol 2016; Qureshi WT, et al. Prognostic value
of extracardiac incidental findings on attenuation correction
cardiac computed tomography. 23: 1266-1274
(226) J Nucl Cardiol 2016; Vitola JV, et al. Outcome of
patients with high-risk Duke tredmill score and normal
myocardial perfusion imaging on SPECT. 23: 1291-1300
(227) J Nucl Cardiol 2016; Matsumoto N, Hirayama A. Clinical
value of high Duke tredmill score with myocardial perfusion
SPECT. 23: 1301-1303
(228) J Nucl Cardiol 2016; Bravo PE, et al. Apparent left
ventricular cavity dilatation during PET/CT in hypertrophic
cardiomyopathy: clinical predictors and potential mechanisms.
23: 1304-1314
(229) J Nucl Cardiol 2016; Folks RD, et al. Optimizing gated
myocardial perfusion imaging processing for phase analysis. 23:
1348-1354
(230) J Nucl Cardiol 2017; Malhotra S, Canty JM. Vasodilator
stress and left ventricular function. 24: 53-56
(231) J Nucl Cardiol 2017; Doukky R, et al. The prognostic
value of regadenoson SPECT myocardial perfusion imaging in
patients with end-stage renal disease. 24: 112-118
(232) J Nucl Cardiol 2017; Hess PL, et al. The prognostic value
of mechanical LV dyssynchrony defined by phase analysis from
gated single-photon emission computed tomography myocardial
perfusion imaging among patients with coronary heart disease.
24: 482-490
(233) J Nucl Cardiol 2017; Gimelli A, et al. Myocardial
ischemia in the absence of obstructive coronary lesion: the role
of post-stress diastolic dysfunction in detecting early coronary
atherosclerosis. 24: 1542-1550
(234) J Nucl Cardiol 2017; Songy B. Detection of
non-obstructive coronary artery disease: is post-stress
diastolic dysfunction assessed by myocardial perfusion imaging a
useful tool? 24: 1551-1554
(235) J Nucl Cardiol 2017; Hulten EA. Does FFRCT have proven
utility as a gatekeeper prior to invasive angiography? 24:
1619-1625
(236) J Nucl Cardiol 2017; Thompson RC, Thomas GS. The EXXERT
study. 24: 1800-1802
(237) J Nucl Med 2019; Slomka PJ, et al. Solid-state detector
SPECT myocardial perfusion imaging. 60: 1194-1204
(238) J Nucl Med 2021; Slomka PJ, et al. Quantitative clinical nuclear cardiology, part 2: evolving/emerging applications. 62: 168-176