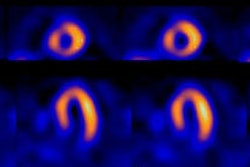
Equilibrium MUGA
Radiopharmaceuticals
The radiopharmaceutical used for the exam can be Tc-tagged RBC's (for labelling methods see GI Bleeding section), or Tc-human
serum albumin. Tc-99m-synthetic polymers have also been
investigated for blood pool imaging [1]. Traditional planar MUGA
exams (using 20 mCi of Tc 99m) provide a radiation exposure
estimated to be equal to 6 to 12 months of natural background
radiation [33].
Technique for
planar imaging:
Dose
The typical dose of technetium used for the exam is 20-25mCi for a rest exam, and 30mCi for an exercise study. A parallel hole medium sensitivity collimator is used and a slight caudal tilt (towards the feet) to the detector head in the LAO view helps in separating the LA from the LV.
Image Acquisition Gated Examination
Most imaging systems acquire a short beat run to determine the average heart rate. The system then divides this R to R interval into a specified number of frames. At least 16 frames per cardiac cycle are required to calculate an accurate ejection fraction. More frames, typically 32 to 60 are required if diastolic information is to be accurately determined. Typically 200,000 to 250,000 counts per frame should be acquired for a rest study to be statistically reliable for evaluation of wall motion.
It is important to always evaluate the ECG strip. In patients with electrical alternans the ECG may only sense every other R-wave. The resultant ventricular volume curve has a "W" shape. With tall peaked T-waves the ECG may sense both the R-wave and the T-wave resulting in the R-T and T-R intervals being recorded as 2 separate beats. This produces volume curves which alternately represent systole and diastole. When these 2 curves are added, the resultant left ventricular volume curve is a flat line. If the T-wave is tall, peaked, and larger than the R-wave it may trigger the ECG gate at end-systole. This gating error will result in the left ventricular volume curve being inverted.
Correcting
Arrhythmias
Arrhythmias will clearly degrade data. When irregular beats account for more than 10% of those recorded, diastolic errors are introduced, with a lesser effect upon the calculated ejection fraction. If more than 30% of the beats are irregular, it is generally advisable not to analyze the data.
Arrhythmiacorrection methods include:
List mode data collection
All data is stored and only desired beats selected for EF calculation.
Post beat filtration
Irregular beats are often followed by one or more other beats which are also irregular. A beat not falling within the pre-selected R-R interval will be recorded, but another beat will not be accepted until they fall back into the appropriate R-R interval.
Dynamic Beat Filtration
The computer evaluates the R-R interval of each beat and records only those within a specified range. Ejection fractions calculated from only the accepted beats do not accurately reflect what the LVEF is during rejected beats.
Technique for gated
blood-pool SPECT imaging:
Images are acquired for a 180? rotation from RAO to LPO in a 64 x 64 matrix using a LEAP or low energy high resolution collimator [12]. For a dual headed camera (in a 90? configuration) 64 views are acquired for 30 seconds per stop, while 32 views for 60 seconds per stop are acquired if using a single-headed camera [12]. Conventional ECG gating can be used with a wide R-R acceptance window (60%), 16 forward gated frames, and standard on-the-fly arrhythmia rejection [12]. Transverse images are reconstructed with a Butterworth filter (cutoff frequency 0.45 - 0.55 Nyquist; order 7) and short axis images are created [12]. Some authors suggest that a 360? rotation provides more accurate results- particularly for LV volume determination [16].
Findings
at Equilibrium MUGA
Wall Motion Evaluation
The anterolateral and apical walls are evaluated using the anterior view; the inferior and posterior walls on the lateral view; and the septal, inferoapical, and posterolateral walls on the LAO view. The walls on the LAO view are supplied by the 3 main coronary arteries: The septum by the LAD, the inferoapical wall by the RCA, and the posterolateral wall by the circumflex.
Visual inspection subjectively grades wall motion as normal, hypokinetic, akinetic, or dyskinetic. A normal segment has a regional shortening of at least 25%. A hypokinetic segment has a regional shortening between 10 and 25%, while an akinetic segment has no shortening, and a dyskinetic segment demonstrates paradoxical systolic motion.
For gated blood-pool SPECT imaging, a volumetric image can be viewed in cinematic format using a transparent grid to define the end-diastolic wall position [12].
Stroke volume
The amount of radioactivity within each ventricle is proportional to the blood volume. The stroke volume image is obtained by subtracting the end-systolic counts from the end-diastolic counts in each pixel. This image is proportional to the net volume changes between diastole and systole in each pixel. In other words, stroke volume is equal to (End diastolic counts - End systolic counts). This number is the numerator used in determination of the ejection fraction.
There are a number of ways to determine the left ventricular volume. One is the 'count based technique': Activity (counts) are measured from a known volume of blood withdrawn from the patient and corrected for decay. Activity (counts) are then determined in the left ventricle by drawing a region of interest around the cavity. Left ventricular volume can then be determined from this data. An attenuation correction factor must be applied to the left ventricular data. Error is introduced because attenuation is actually highly variable from patient to patient.
Left
Ventricular Ejection Fraction
The normal LVEF is typically between 60-70%. A LVEF less than 50% is definitely abnormal (In children, LVEF should be greater than 50%, and RVEF should be greater than 41%). The LVEF calculated by the gated examination is fairly reproducible, but a change of 5 to 7 percentage points is probably necessary before one can confidently claim a significant change over time. The counts within the ventricle are proportional to the volume and, with correction for background, accurate measurements of LVEF can be obtained:
EF = (End diastolic counts - End systolic counts) / (End diastolic counts - Background)
Background correction cancels out in the numerator. Background activity can affect the calculated LVEF. High background will falsely elevate EF, while low background will falsely lower the calculated EF. The left atrium may partially overlap (posteriorly) the left ventricle in the LAO projection (best septal view) [12]. The partial inclusion of the left atrium in a left ventricular ROI has been shown to decreased LVEF because, relative to diastole, counts in the filled left atrium contribute to activity within the ventricle at end-systole [12]. This effect seems to be greatest in patients with better left ventricular function- patients with higher LVEF's have smaller end-systolic volumes which means that the relative contribution from left atrial counts will be more significant than in patients with large end-systolic volumes [12].
ECG gated blood-pool SPECT imaging has the benefit of a tomographic perspective that can permit isolation of the left and right ventricles without overlap of other cardiac chambers [12].There is no need to search for the best septal projection image [13]. The technique can provide simultaneously accurate LV and RV ejection fraction and volumes [9]. A 360? orbit has been suggested as providing the most accurate results [16]. SPECT left ventricular ejection fractions tend to be somewhat higher than planar LVEF's (about 8 percentage points higher)- possibly due to the improved exclusion of left atrial activity from the ventricular ROI [12]. The difference between planar and SPECT LVEF's is greatest for patients with higher ejections fractions (see above for discussion of effect of left atrial counts in LVEF determination) [12]. Although reproducibility appears good [17], one drawback of the gated SPECT MUGA exam is that there may be poor exam reproducibility in patients with heart failure [13].
Many treatment algorithms in cardiology utilize the LV ejection fraction in clinical decision making [10]. For patients with 3 vessel coronary artery disease or LAD stenosis of greater than 70%, those with an LVEF of less than 50% have a statistically significant survival benefit if treated with surgical revascularization compared to medical therapy [10].
Left Ventricular Output
LV output is derived from the product of heart rate, ejection fraction, and end-diastolic volume.
Phase Analysis
The phase image presents the ventricular contraction sequence. Phase imaging can be useful in detecting asynchronous ventricular contraction. The timing of regional ejection (contraction) in each cardiac pixel appears on the phase image. This allows detection of the frame in which each portion of the ventricle maximally ejects its blood volume. If two pixels defining the heart reach a maximum (end-diastole) or a minimum (end-systole) at the same time, then there is no phase difference between the two. The phase image readily identifies abnormal timing of ventricular contraction (such as tardykinesis [late contraction] and dyskinesis,). The atria and ventricles are 'out of phase'- they contract at different times approximately 180 degrees apart.
In phase analysis, areas which change activity over time are assigned a color. The activity in the lungs and spleen remain relatively constant and they are therefore not assigned a color and remain black. Areas of akinesis also demonstrate no change and also appear black. Regions of tardykinesis have delayed phase (contraction), or an increased phase angle. Areas of ventricular dyskinesis are closer in phase to atrial contraction, and will therefore be assigned a color similar to the atria. In the presence of a left bundle branch block, the ventricles will appear slightly out of phase due to delayed LV contraction resulting in a broadening of the phase histiogram peak.
Amplitude (Stroke volume) Analysis
The amplitude image shows the magnitude of blood ejected from each pixel within the ventricular chamber. Lower values are displayed for those regions of the heart associated with hypo- or akinesis. The amplitude image is calculated from the entire time-activity curve.
The stroke volume images show color coded net regional counts after subtracting the end-systolic image from end-diastolic image (i.e.: the magnitude of the regional volume ejected in systole [15]).
Paradox images depict end-diastole subtracted from end-systole. Functional images such as these are generally less sensitive than visual analysis in detecting regional dysfunction as errors due to anterior translocation of the heart with contraction or patient motion can be introduced.
Gated
Exercise Examination
The examination most commonly consists of supine exercise performed on a bicycle. Supine stress produces different physiologic responses compared to upright exercise. The elevation in blood pressure is typically more than when erect and the elevation in heart rate is typically less than when erect. The resting end-distolic and end-systolic volumes are also larger when supine and increase less with exercise when compared to erect exercise. Exercise begins at a 25 or 50 watt baseline and increases in 25 watt increments every 3 minutes. The image is acquired during the last 2 minutes of the exercise stage. Generally, for the exercise exam a high sensitivity collimator is used to obtain higher count rates (at the expense of spatial resolution).
The exam is considered abnormal and indicative of coronary artery disease if LVEF remains unchanged or decreases with exercise (normally LVEF will increase by 5 to 7 percentage points with exercise) or if a new regional wall motion abnormality develops. Lack of the expected increase in LVEF can be seen normally in:
- Patients over the age of 65y
- Females: Females tend to have a smaller increase
in EF with exercise, while having a greater increase in
the end-diastolic volume compared to males due to a
greater degree of left ventricular dilatation. Exercise
conditioning will also increase the end-diastolic volume
attained during maximal exertion.
- Patients with resting ejection fractions greater
than 65%
- Patients who fail to achieve adequate cardiac
stress: Patients being medicated with beta blockers
The combination of an exercise EF less than 6% of that predicted and the presence of exercise-induced wall motion abnormalities provide a sensitivity of 89% and a specificity of 79% for the diagnosis of coronary artery disease. [2] Exercise EF is an excellent predictor of the patients risk for cardiovascular death. Patients with exercise EF's of less than 35% are at high risk for future cardiac events, whereas an exercise EF of greater than 50% is associated with a low probability of subsequent cardiac events [2]. In another study, patients with an exercise EF less than 30% had a 5 year survival of 60%, compared to a 97% survival in patients with an exercise EF greater than 30% [3].
Early exercise radionuclide angiography can also be used to identify patients who are at high risk for restenosis following successful angioplasty. In patients with a normal exam 1 month following the procedure, no patient developed restenosis, while 42% of patients with an abnormal exam subsequently restenosed [4].
DiastolicVentricular
Function
Diastolic filling is dependent on properties of the LV such as myocardial relaxation rate, elastic recoil, and diastolic stiffness [19]. The pathophysiology of diastolic dysfunction is largely related to impaired LV relaxation and increased myocardial stiffness [19]. Aberrations in diastolic function can be the earliest indicator of left ventricular dysfunction/early heart failure. Patients with diastolic dysfunction may have preserved ejection fractions [19]. Clinical recognition of diastolic dysfunction is important since routine therapy for systolic failure with inotropic agents or vasodilators will not improve diastolic function, and may be deleterious. Beta blockers produce little or no change in diastolic filling. Calcium channel blockers such as verapamil, are more effective in the treatment of diastolic failure and have been shown to increase peak filling rate.
In order to accurately measure diastolic events, a large number of acquisition frames must be used (32 to 64). It is also important to use narrow gating tolerance- all beat lengths should be within about 5% of one another. Longer acquisition times are required to achieve less statistical fluctuations which would affect curve fitting results. The duration of diastole clearly depends on the cardiac cycle length. Variability in the R-R interval will greatly affect the resulting volume curve and consequently the accuracy of all diastolic parameters measured.
Diastole can be divided into 3 phases:
- Isovolumetic relaxation
- Rapid ventricular filling- in the normal
ventricle early diastole accounts for 60-80% of
ventricular filling [19].
- Passive ventricular filling
(the last being interrupted by atrial systole just prior to the next ventricular systole.) The atrial contribution to ventricular volume varies from 10 to 25%.
Rapid Ventricular Filling Phase/Peak Filling Rate
This phase follows the opening of the mitral valve. About 60-80% of the ventricular volume enters the left ventricle during the rapid filling phase. The peak filling rate is the most widely used parameter of diastolic function and represents the maximum value of the first derivative of the time-activity curve [21]. It is expressed in units of end-diastolic volumes (or sometimes stroke volumes) per second [21]. The most important determinant of the peak filling rate is the rate at which active left ventricular relaxation occurs (a reflection of left ventricular compliance), but it is also affected by the pressure gradient between the left atrium and ventricle. Peak filling rates (PFR) increase with increasing heart rate and inotropic intervention.
Normal values for PFR will vary between systems, but generally PFR should be greater than or equal to 2.0-2.5 end-diastolic volumes per second. Diastolic filling is age dependent and even in the absence of overt heart disease, peak filling rates decline with age. The time to peak filling rate (TPFR) is the time from end-systole until the peak filling rate is achieved, expressed in milliseconds [21]. The TPFR should be less than 180 msec. The E/A ratio should be greater than 40 [19]. In diastolic dysfunction there is a decrease in peak filling rate, a prologation of the time ot PFR, and a reduction in the E/A ratio [19].
Entities
Associated with a Decreased Peak Filling Rate (PFR) Include:
1- Coronary artery disease
Rest imaging in patients with CAD can detect subclinical diastolic dysfunction. A depressed peak filling rate can be seen in about 50% of patients with CAD, and up to 85% of patients with a history of prior myocardial infarction [5].
2-
Congestive Heart Failure
3- Restrictive and dilated cardiomyopathies
4- Hypertrophic Cardiomyopathy
Decreasesmyocardial compliance
5- Valvular heart disease
Aortic regurge and aortic stenosis
6- Rejection following cardiac transplant
Abnormalities in diastolic filling after cardiac transplantation may provide a marker of early rejection.
7- Hypertension
Isolated diastolic dysfunction in patients with HTN may be an early indicator of hypertensive heart failure.
8- Medications
- Nitroglycerine: Given acutely
- Chemotherapy: Doxorubicin
- Beta-blockers: Beta-blockers have no effect on
the PFR
Entities Associated with an Increased PFR include:
- Constrictive pericarditis
(occasionally)
- Mitral regurge: Due to accentuation of
mitral flow in early diastole
- Medications: Calcium channel
blockers
Valvular Heart Disease
Gated equilibrium examinations have little role in the evaluation of stenotic valvular lesions. Its principal role in valvular heart disease is the assessment of left sided regurgitant lesions.
Aortic Insufficiency
These patients tend to have a normal resting ejection fraction due to an increased left ventricular end diastolic and stroke volume. Exercise induced left ventricular dysfunction appears before resting dysfunction in these patients. However, left ventricular dysfunction manifested by a decrease in LVEF with exercise is not necessarily an indication for surgery in these patients and does not predict lack of reversal of LV dysfunction after aortic valve replacement. However, patients who demonstrate resting left ventricular dysfunction have a greater operative risk. Therefore, if exercise induced LV dysfunction is mild, these patients can probably continue to be managed medically. If the dysfunction is severe, however, these patients should be considered surgical candidates.
LV dysfunction manifested by an abnormal LVEF at rest is an indication to consider surgical valve replacement, especially in symptomatic patients. Resting LVEF is also a major predictor of postoperative survival with a 96% 5 year survival in patients with a normal preoperative LVEF, compared to 60% in patients with an abnormal value [6].
LV end-systolic volume can be used to risk stratify patients with aortic insufficiency pre-operatively [10]. A pre-operative end-systolic volume of greater than 60 mL/m2 predicts a high risk for perioperative cardiac death [10].
Aortic Stenosis
Early in the course of aortic stenosis, patients develop left ventricular hypertrophy, and the LVEF is often normal or elevated (due to a decreased end diastolic volume). In patients with aortic stenosis, an increase in afterload is imposed by the stenosis (which restricts left ventricular emptying), and this may cause a decrease in LVEF during exercise. Exercise induce ischemia may also occur due to insufficient coronary blood flow which is limited by the stenosis. Patients with aortic stenosis and an abnormal resting LVEF have a less favorable prognosis following aortic valve replacement.
Anthracyclines/Adriamycin
(Doxorubicin) or other chemotherapy induced Cardiotoxicity
(See also Myoscint Imaging)
Chemotherapy with anthracyclines (such as doxorubicin,
daunorubicin, epirubicin, and idarubicin) produce a dose-dependent impairment in
left ventricular function secondary to toxic effects on
cardiac myocytes [11,14,23,29]. The pathophysiology of
anthracycline cardiotoxicity is controversial, but are likely
secondary to the induction of free radicals that damage the
myocardiocyte cell membranes leading to cell death and
fibrosis [29]. Anthracyclines also inhibit DNA repair enzymes
and block messages that control myocardial contractility [29].
Anthracyclines are used to regularly treat a variety of
malignancies including breast cancer, leukemia, lymphoma,
neuroblastoma, ovarian cancer, gastric cancer, and sarcoma
[23]. Anthracyclines can cause acute and late/chronic cardiac
injury [29]. Acute cardiac toxicity from the agent is rare
(less than 1% of patients) occuring within hours to days after
the drug was administered. The toxicity is transient and
should improve after about 1 week.
Chronic adriamycin cardiomyopathy is generally noted to
occur approximately 30 days, up to 6 months, after the last
dose. The majority of patients develop cardiotoxicity within
the first year after receiving the agent, but there is large
inter-individual variation in susceptibility to doxoruicin
cardiotoxicity [33]. Cardiac toxicity is usually dose
dependent (cumulative dose is the most important determinant
for cardiotoxicity [33]) and the incidence increases beyond a
cumulative dose of 400 mg/m2
[18]. Although there is no threshold below which
toxic effects do not occur, the probability of cardiotoxicity is 3-5% or less at a
dose of 400 mg/m2, 7-26% at 550 mg/m2,
and 18-48% at doses of 700 mg/m2 [18,23,29].
However, there are patients who receive doses exceeding 1g/m2 that do not develop cardiotoxicity
indicating that other factors also contribute to the
development of cardiotoxicity [23]. Pre-existing conditions
such as age greater than 70 years, underlying CAD, combination
chemotherapy (cyclophosphamide, tratuzumab, tyrosine kinase
inhibitors, and taxanes), and mediastinal/thoracic
radiation therapy increase the risk for developing cardiotoxicity [14,20,29,33]. Other
authors also note a higher incidence of late onset cardiotoxicity in children [22]. There
is a higher incidence of cardiotoxicity in breast cancer
patients that received a regimen that involved concurrent
herceptin and anthracycline administration, compared to those
receiving herceptin after completing anthracyline treatment
(27% vs 7%) [23].
A drop in LVEF suggests doxorubicin
cardiotoxicity. In patients with
pretreatment LVEF's above 50%, treatment is usually
discontinued if the ejection fraction falls more than 10
percentage points OR to a value below 50% [18,23]. However, as long as the resting LVEF
remains above the lower limit of normal, some authors suggest
it is safe to continue doxorubicin therapy [11]. In patients with a baseline LVEF less
than 50%, treatment is usually discontinued if the ejection
fraction falls more than 10 percentage points or drops below
30%. Doxorubicin treatment is generally not performed in
patients with baseline LVEF's below 30% [7,18].
Other guidelines suggest cardiotoxicity with a reduction in
LVEF >/= 5% to a value below 55% with symptoms of heart
failure [31].
Serial evaluation of LVEF at rest using the MUGA exam is an effective means to monitor patients during doxorubicin chemotherapy [11]. LVEF should be obtained at baseline and repeated during therapy and after completion if doses exceed 300mg/m2 or if another potentially cardiotoxic agent is to be administered [20]. Guidelines for serial monitoring of doxorubicin cardiotoxicity [18]:
1- Baseline exam before initiation chemotherapy or prior to 100 mg/m2. Followup exams performed 3 weeks after the indicated last dose and before the next planned treatment.
2- Patients with baseline LVEF ≥ 50%; second study after 250-300 mg/m2; repeat exam after 400 mg/m2 in patients with known heart disease, hypertension, radiation exposure, ECG findings, or cyclophosphemide therapy; or repeat exam after 450 mg/m2 in the absence of risk factors.
3- Patients with baseline 30-50%: perform repeat study prior to each dose [33].
Following discontinuance of the
therapy, the left ventricular dysfunction is generally
irreversible [14], but myocardial function will stabilize in
most patients [11]. Some patients will improve, but a small
percentage will continue to deteriorate. This is especially a
problem in children who have a tendency to develop CHF as
adolescents. Further doxorubicin administration once the
resting LVEF drops below normal runs the risk of precipitous
impairment in LV function associated with serious,
life-threatening heart failure [11]. Because doxorubicin also
commonly produces an acute transient deterioration in
ventricular function (acute cardiotoxicity),
assessment of LVEF should be timed at least 10 to 14 days
after the last dose of chemotherapy [11].
The actual incidence of cardiotoxicity may be underestimated as long term followup studies have suggested a 63% prevalence of LV dysfunction after more than 10 years in patients that received more than 500 mg/m2 cummulative dose, compared to an 18% prevalence in patients that had received less than 500/m2 [23].
Newer chemotherapeutic agents are
also associated with cardiotoxicity
[22].
Trastuzumab (Herceptin) is a humanized monoclonal antibody directed against human epidermal growth factor receptor type 2 (HER2/neu) that can be over-expressed in breast cancers [22,23]. HER2 receptors are expressed by cardiac myocytes and exert cardioprotective effects [32]. The overall incidence of trastuzumab-induced cardiotoxicity varies, but ranges from 2-7% for monotherapy, and 2-13% for trastuzumab combined with paclitaxel (other authors state cardiotoxicity occurs in up to 10% of patients [22]) [31]. Combined concurrent use of anthracyclines and trastuzumab are at additional risk for cardiotoxicity [22,23,30] and the incidence of cardiotoxicity can be as high as 27% when the agent is used in combination with anthracyclines [31]. Trastuzumab-induced cardiotoxicity is not dose dependent, is not associated with cardiomyocyte ultrastructural changes, and is typically of more rapid onset than that associated with anthracyclines [31,32,33]. The cardiotoxicity due to herceptin is considered to be reversible within a few months of terminating therapy if a prompt diagnosis is made with discontinuation of herceptin and initiation of medical treatment for heart failure [23,31,32]. The agent can also be safely re-administered after recovery of LVEF and does not necessarily result in recurrence of LV dysfunction [31,33]. Unlike anthracycline induced cardiotoxicity, diastolic dysfunction does not appear to precede systolic dysfunction in patients with trastuzumab cardiotoxicity [31].
Taxanes
are agents used in the treatment of lung, breast, and ovarian
cancer [22]. These agents impair the normal microtubular transport system in cardiomyocytes, resulting in a failure
to store free fatty acids in the lipid pool of the cytosol [22]. As a result, uptake of
free fatty acids by the mitochondria may be reduced [22].
Tyrosine kinase inhibitors may also
cause LV dysfunction [33]. Up to 11% of patients receiving
sunitinib can have clinical manifestations of CHF or a
decrease in LVEF of at elast 20% to an LVEF < 50% [33].
Other means to detect chemotherapy
induced cardiotoxicity:
A problem with relying on resting
LVEF alone for the assessment of cardiotoxicity is that many
patients will develop histologic evidence of
anthracycline-related changes without resting LV dysfunction
[23].
Theorectically, diastolic filling
abnormalities should occur prior to a drop in LV systolic
function [23]. Diastolic parameters can be obtained from the
MUGA exam including peak filling rate and the time to peak
filling rate [23]. Changes in diastolic parameters, however,
to not appear to be able to predict subsequent systolic
dysfunction [23,26,27].
Adrenergic cardiac imaging has also been studied
to evaluate for chemotherapy induced cardiotoxicity. Decreased
myocardial MIBG uptake can be seen following doxorubicin
therapy, with limited morphologic damage [24]. Decreased MIBG
uptake follows a dose dependent decline with about 25% of
patients demonstrating some decrease in MIBG uptake at
cumulative doses of 240-300 mg/m2 [24]. Decreased MIBG uptake
precedes deterioration of ejection fraction [24,25]. Evidence of sympathetic damage can
be used to select patients at risk of severe functional
impairment and who may benefit from cardioprotective
agents or changes in the schedule of antineoplastic
drugs [24].
On FDG PET, it has been suggested that a low
baseline myocardial FDG uptake with progressively increased
uptake over time, suggests an increased risk for late cardiac
chemotherapy induced abnormalities [34].
Delayed contrast MR imaging can demonstrate areas
of focal or linear mid-myocardial enhancement in patients with
chemotherapy induced cardiotoxicity that have already developed
LV dysfunction [23].
Post-Myocardial
Infarction Prognostic Evaluation
The rest exam can be used to identify patients following MI that are at high risk for subsequent cardiac events. In general, a patients outcome is related to the extent of left ventricular damage and the extent and degree of residual ischemia. The resting LVEF is a reflection of the extent of myocardial damage and has been identified as the single most important factor in determining survival following a myocardial infarction [8].
One year cardiac mortality is inversely related to the resting LVEF in a continuous fashion. A resting LVEF of less than 30% is associated with a markedly increased risk (30%) of subsequent cardiac morbidity and mortality within the first year post event (an EF of less than 20% has a mortality approaching 50%). Preserved left ventricular function, however, is associated with only a 5% risk of death during the first year post MI.
Another major determinant of survival after recovery from a myocardial infarction is LV end-systolic volume. A LV end-systolic volume of less than 70 mL is associated with a low mortality rate, even in patients with severe perfusion abnormalities [10].
Evaluation of Response to Drug Therapy
Resting LVEF may also prove beneficial in the assessment of response to drug therapy. Recently, enalapril has been shown to decrease end-diastolic and end-systolic volumes in patients with symptomatic LV dysfunction. Because patients with progressive LV dilatation and/or depression in ejection fraction have a high mortality rate, a reduction in the volumetric indices may translate to improved survival.
First-Pass MUGA
Radiopharmaceuticals
Any Technetium-labeled agent can be used for a first-pass exam.
Tc-Pertechnetate
Cancombine the exam with an equilibrium study by giving Sn-PYP prior to the injection.
Tc-DTPA
Rapid renal clearance decreases the biologic half-life of the agent and permits rapid reimaging.
Tc-Sestamibi
Cancombine with myocardial perfusion study.
Technique
Inject right external jugular vein when possible, but the antecubital vein can be adequate. A tight bolus is required (less than 2 sec. duration). A broken bolus is even worse than a spread out injection. The bolus is flushed with 10-20cc of normal saline. Images are performed in the anterior or RAO projection to best separate the right atrium and ventricle. A multicrystal camera is preferred because it has a count rate capability of up to 500,000 to 1,000,000 cps, whereas a single crystal camera can only count up to 150,000 cps.
High count rates are essential for statistical reliability. There should be a peak count density during the right ventricular phase of at least 3000-4000 counts per 50 msec frame. Images are acquired at 20 to 25 msec intervals with a total of 800,000 counts per second for a total of 30 to 60 seconds after injection using a 20 mCi dose (with a single crystal camera the count rate decreases significantly) [2]. During first-pass transit, the tracer bolus is temporally and anatomically separated in each ventricle. Consequently, the performance of the left and right ventricle can be measured separately.
Findings on the First-Pass MUGA Exam:
Right Ventricular Ejection Fraction
Limited information regarding right ventricular function can be obtained from the equilibrium exam. The normal RVEF is typically 5-10% less than LVEF, this is because total right ventricular volume is usually slightly greater than the left. The RVEF may be obtained from the equilibrium exam using the lower 2/3's of the RV from the best septal view. The upper 1/3 is not used because the right atrium lies behind it. However, the RVEF is best determined from the first-pass exam, because background subtraction is not required [29]. A decreased RVEF has been identified as an independent predictor of increased risk for mortality and morbidity in patients with chrnoic ischemic heart disease [29].Left to Right
Shunt
The anterior view is generally acceptable for shunt studies. In normal individuals the tracer bolus passes from the right ventricle through the lungs to the left ventricle. At least 8 seconds pass before a small recirculation peak is observed after the tracer has passed through the systemic circulation and returned to the heart. A left to right shunt at the ventricular level will produce early recirculation to the right heart and early reappearance or persistence of activity in the lungs. Data recorded over the lung at a site remote from the heart provides the most accurate quantitation of shunt flow.
Shunted tracer interrupts the expected exponential decline of the lung count curve and counts may actually increase sufficiently to cause a second curve peak. The size of the early recirculation peak in the pulmonary time activity curve is proportional to the size of the shunt. More commonly the recirculated counts simply blend with the initial counts and the multiple rapid recirculations cause the curve to remain relatively flat with a high background. Gamma variate function approximations over the initial and recirculation segments of the pulmonary transit curve can accurately quantify left to right shunts between 1.2 to 3.5:1. The success of this technique is dependent upon a tight bolus.
Area A
Pulmonary/Systemic Ratio = Qp/Qs = -------------------------
(Area A - Area B)
...where 'Area A' equals the area under the first peak, and 'Area B' equals the area under the second peak.
Right to Left
Shunt
Counts detected in the aorta soon after tracer appearance in the right heart and prior to normal lung activity confirm the presence of right to left shunting. Data used to quantify the shunt must be recorded from a site peripheral to the heart such as the carotid arteries due to scatter from the lungs affecting counts from more central sites.
Tc-MAA can also be used to document the presence of a right to left shunt. Normally, only 3 to 6% of the injected Tc-MAA will bypass the pulmonary vasculature. The magnitude of the right to left shunt can be expressed as:
(Total Body Counts - Total Lung Counts)
Qs = ------------------------------------------------------------- x 100%
Total Body Counts
A surgical systemic to pulmonary artery shunt will dilute the concentration of Tc-MAA in the pulmonary artery at the anastamosis, but will increase the total number of particles delivered to the lungs. Thus, when a shunt is successful, the concentration of Tc-MAA in the lung that receives the shunt will decrease, while the overall amount of tracer deposited in the lungs increases, and the calculated right to left shunt also decreases.
Regurgitation Index (Stroke Volume Ratio)
The stroke volume ratio has been routinely calculated as left ventricular stroke counts divided by right ventricular stroke counts. The ratio is a non-invasive determination of the severity of mitral or aortic regurge. The calculation is based upon the assumption that in normal individuals the right and left ventricular stroke counts should be equal.
LV stroke cts (ED cts - ES cts [LV])
SV Ratio = -------------------- = ---------------------------------
RV stroke cts (ED cts - ES cts [RV])
(LV stroke cts - RV stroke cts)
Regurgitant Fraction = -------------------------------------------- x 100%
LV stroke cts
Regurgitant lesions of the mitral and aortic valve are associated with volume overload in the left ventricle because it ejects not only blood pumped forward from the right ventricle, but also regurgitant blood. The ratio of stroke counts should be near unity in patients without evidence of left sided regurge. Because right ventricular stroke volume can be underestimated due to right atrial overlap at end systole the ratio may normally exceed 1:1. For this reason the upper limit of normal is usually considered 1.2:1. The ratio will be elevated in patients with mitral or aortic regurge because the stroke volume of the left ventricle exceeds that of the right ventricle. An SVR > 2 indicates moderately severe regurge, while an SVR > 3 indicates severe regurge. The stroke volume ratio has been shown to agree with the angiographic qualitative assessment of the amount of reflux. In patients with tricuspid or pulmonic insufficiency the RV stroke counts will be greater than the LV stroke counts and the ratio will decrease (less than 0.7 in patients with clinically significant tricuspid regurge).
The major limitation of the stroke volume ratio is the presence of right atrial and right ventricular overlap in most patients, thus, even normal patients may show elevated stroke volume ratios. A LAO view with significant caudal-cranial angulation may help to minimize this overlap. The ratio is also less accurate in patients with baseline LVEF's below 30%, coexistant right-sided regurgitation, arrhythmias, LV aneurysms, and mitral valve prolapse.
The ratio is not routinely used in the evaluation of left to right shunts because small shunts can be missed. In the setting of a VSD the ratio would be expected to increase. Results in these patients, however, should be interpreted with caution as diastolic shunting can produce right ventricular volume overload and a decreased stroke volume ratio.
In patients with tricuspid regurge there is increased hepatic blood volume during systole due to back flow in the hepatic veins. This cyclic variation in counts over the liver can be detected on the gated exam.
REFERENCES:
(1) AJR 1998; Callahan RJ, et al. Preclinical evaluation and phase
I clinical trial of a Tc-99m-labeled synthetic polymer used in
blood pool imaging. 171: 137-143
(2) Radiol Clin North Am 1993; Borges-Neto S, Coleman RE. Radionuclide ventricular function analysis.31: 817-830
(3) J Am Coll Cardiol 1988. McKenna WJ, et al. Arrhythmia and prognosis in infants, children and adolescents with hypertrophic cardiomyopathy. 11: 147-153
(4) Am J Cardiol 1988; O'Keefe JH, et al. Usefulness of early radionuclide angiography for identifying low-risk patients for late restenosis after percutaneous transluminal coronary angioplasty. 61: 51-54
(5) J Nucl Med 1983; Miller TR, et
al. Analysis of cardiac diastolic function: application in
coronary artery disease.
24: 2-7
(6) Circulation 1985; 72: 1244
(7) J Nucl Med 1990; Jain D, Zaret BL. Antimyosin cardiac imaging: will it play a role in the detection of doxorubicin cardiotoxicity? 31: 1970-74
(8) New Engl J Med 1993; Zaret BL, Wackers FJ. Nuclear cardiology. 329: 855-863
(9) J Nucl Med 2001; Daou D, et al. Electrocardiographically gated blood-pool SPECT and left ventricular function: Comparitive value of 3 methods for ejection fraction and volume estimation. 42: 1043-1049
(10) J Nucl Med 2001; Cacciabaudo JM. Gated cardiac SPECT: Has the addition of function to perfusion strengthened the value of myocardial perfusion imaging? 42: 1050-1052
(11) JACC 1995; Rictchie JL, et al. Guidelines for clinical use of cardiac radionuclide imaging. 25: 521-547
(12) J Nucl Med 2001; Groch MW, et al. Planar imaging versus gated blood-pool SPECT for the assessment of ventricular performance: a multicenter study. 42: 1773-1779
(13) J Nucl Med 2003; Wright GA, et al. Left ventricular ejection fraction and volume from gated blood-pool SPECT: comparison with planar gated blood-pool imaging and assessment of repeatability in patients with heart failure. 44: 494-498
(14) J Nucl Cardiol 2003; Mitani I, et al. Doxorubicin cardiotoxicity: prevention of congestive heart failure with serial cardiac function monitoring with equilibrium radionuclide angiocardiography in the current era. 10: 132-39
(15) J Nucl Cardiol 2003; Botvinick EH. Scintigraphic blood pool and phase image analysis: the optimal tool for the evaluation of resynchronization therapy. 10: 424-428
(16) J Nucl Cardiol 2005; Adachi I, et al. Comparitive study of quantitative blood pool SPECT imaging with 180? and 360? acquisition orbits on accuracy of cardiac function. 12: 186-194
(17) J Nucl Cardiol 2005; Akinboboye O, et al. Accuracy of radionuclide ventriculography assessed by magnetic resonance imaging in patients with abnormal left ventricles. 12: 418-427
(18) J Nucl Cardiol 2006; Panjrath GS, Jain D. Monitoring chemotherapy-induced cardiotoxicity: role of cardiac nuclear imaging. 13: 415-426
(19) J Nucl Cardiol 2010; Multi-modality imaging of diastolic function. 17: 316-327
(20) Radiology 2011; Tossisi JM, et al. CT findings of chemotherapy induced toxicity: what radiologists need to know about the clinical and radiologic manifestations of chemotherapy toxicity. 258: 41-56
(21) J Nucl Cardiol 2011; Shirani J, Dilsizian V. Nuclear cardiac imaging in hypertrophic cardiomyopathy. 18: 123-134
(22) J Nucl Med 2011; de Geus-Oei LF, et al. Scintigraphic
techniques for early detection of cancer treatment-induced cardiotoxicity. 52: 560-571
(23) J Nucl Cardiol 2012; Jiji RS, et al Non-invasive imaging and
monitoring cardiotoxicity of cancer therapeutic drugs. 19: 377-388
(24) J Nucl Med 2001; Carrio I. Cardiac
neurotransmission imaging. 42: 1062-1076
(25) J Nucl Cardiol
2002; Patel AD, Iskandrian AE. MIBG imaging. 9: 75-94
(26) Heart 2004; Dorup
I, et al. Prospective longitudinal assessment of late
anthracycline cardiotoxicity after childhood cancer: the role of
diastolic function. 90. 1212-1216
(27) Eur J Nuc Med 1988;
Parmentier S, et al. Assessment of
left ventricular diastolic function in patients receiving
anthracycline therapy. 13: 563-567
(29) Radiographics
2013; Walker CM, et al. Cardiac complications of oncologic
therapy. 33: 1801-1815
(32) J Nucl
Cardiol 2016; Schwartz RG, Venci
N. Can serial changes of diastolic dysfunction signal
incremental risk of chemotherapy-induced heart failure missed by
the timing of declining LV ejection fraction? 23: 824-832
(34) J Nucl Med 2017; Bauckneht M, et al. Doxorubicin effect on myocardial metabolism as a prerequisite for subsequent development of cardiac toxicity: a translational 18F-FDG PET/CT observation. 58: 1638-1645