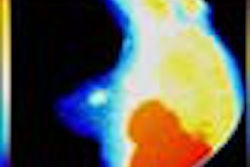
Researchers at the University of California, San Diego, Cancer Center have received clearance from the Food and Drug Administration to begin investigational clinical evaluation of a radiolabeled agent designed to assist in the more accurate surgical management and diagnosis of breast cancer patients.
The agent, to which a radioactive isotope is attached, has shown considerable ability in preclinical studies to accurately identify lymphatic tissue, according to gamma-guided surgery firm Neoprobe. Dublin, OH-based Neoprobe holds an option agreement with the Regents of the University of California to exclusively license the proprietary UCSD agent for diagnostic use.
Patient enrollment in the randomized clinical study will begin shortly, according to Neoprobe. Dr. Anne Wallace, an assistant professor of surgery and director of the Breast Care Unit at UCSD Cancer Center, is the principal physician investigator evaluating the agent in breast cancer patients. Dr. Wallace's Phase I clinical study, funded with a grant from the Susan G. Komen Breast Cancer Foundation, will evaluate the agent's ability, when used with Neoprobe's hand-held gamma radiation detection instruments, to accurately identify tumor draining lymph nodes.
By AuntMinnie.com staff writersMarch 8, 2001
Related Reading
Radiolabeled breast cancer agent to begin clinical evaluation, February 14, 2001
Copyright © 2001 AuntMinnie.com