North American Scientific has withdrawn its Apomate radiopharmaceutical investigational new drug (IND) application from the Food and Drug Administration. The Chatsworth, CA-based firm said it has requested a meeting with the agency to resolve issues of protocol design and related matters for planned U.S. studies of Apomate (Tc-99m hynic-annexin).
In order to incorporate expected changes in laboratory validation testing and clinical protocol into future clinical studies, NAS withdrew the recently filed IND. The company said it would refile the IND shortly after completion of the FDA meeting.
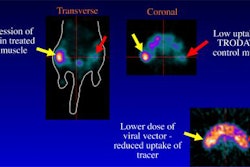
In other NAS news, the company reported that two presentations at this week's annual meeting of the Society of Nuclear Medicine reported favorable results for Apomate in imaging apoptosis. In evaluating preclinical models of liver cancer, researchers concluded that tumor uptake of Apomate correlated well with microscopic detection of apoptosis.
In addition, investigators found that tumor uptake of Apomate was independent of blood flow, a finding that supports the specific molecular basis of annexin imaging, NAS said.
By AuntMinnie.com staff writersJune 18, 2002
Related Reading
NAS loss widens on one-time charges, May 22, 2002
Philips, Theseus team up, February 21, 2002
NAS moves into the black, February 20, 2002
NAS revenues grow while income dips, December 11, 2001
NAS unit begins clinical Apomate trials, November 30, 2001
Copyright © 2002 AuntMinnie.com