CT-guided radiofrequency catheter ablation is being performed with increasing frequency to treat recurrent or refractory atrial fibrillation (AF), a common cardiac dysrhythmia that is producing significant morbidity and mortality in a growing population of older adults. There are an estimated 2.5 million AF cases in the U.S., representing 5% of all adults 65 and older.
In a comprehensive talk at the 2004 International Symposium on Multidetector-Row CT in San Francisco, Dr. Joan Lacomis, associate professor of radiology at the University of Pittsburgh Medical Center, described the evolution of the ablation procedure at her institution, which performs them frequently. She also discussed the importance of good preprocedural imaging with MDCT.
RFCA is a delicate procedure that is undertaken to short-circuit errant electrical signals in the heart that can produce atrial fibrillation. Its success is highly dependent on detailed MDCT images that enable the electrophysiologist to understand the complex anatomy of the distal pulmonary veins and the left atrium, Lacomis said. As a result, the quality of MDCT-reconstructed images can directly affect outcomes. Neither fluoroscopy nor echocardiography are capable of adequately depicting the anatomy, and MR angiography is hampered by its unsuitability for use with pacemakers, which are seen in about a third of AF patients.
In normal individuals, a specialized bundle of neurons known as the SA (sinoatrial) node "paces the heart by generating the electrical signal that travels through the right atrium into the left atrium via Bachmans bundle, on to the ventricles via the intraventricular node, and the bundle of His," Lacomis explained. "If the conduction system is intact, we expect synchronous atrial conduction -- contraction followed by synchronous ventricular contraction. Conversely, in atrial fibrillation, multiple ectopic electrical foci fire independently of the SA node. Instead of receiving one electrical signal, the AV (atrio-ventricular) node is bombarded by as many as 300 discharges per minute."
The jumbled electrical signals prevent the atria from contracting properly. The resulting heart rate, depending on the refractoriness of the atrioventricular node, can vary from 30 to 300 bpm -- either too fast or too slow to maintain adequate cardiac output, she said. Hemodynamic compromise results from the loss of the so-called atrial kick, and the formation of thrombi heightens the risk of stroke in AF patients. As a result, Lacomis said, in 2004 AF remains a significant cause of morbidity and mortality in the U.S.
The role of pulmonary veins
The muscular sleeves of the distal pulmonary veins have recently been identified as a primary source of ectopic electrical foci, she said. Indeed, the left superior pulmonary vein alone accounts for half of all ectopic beats resulting in atrial fibrillation. RFCA works by electrically isolating the conduction pathways through ablation of the distal pulmonary veins and posterior left atrium.
"When originally described, ablations were directed at site-specific arrhythmogenic foci, and only a point ablation would be done," she said. "At our institution, and several others now, the tendency is to increase the number of ablated lesions further. There is believed to be a certain volume of atrial tissue that is required to sustain atrial fibrillation."
Lacomis' institution now routinely performs what are known as pulmonary inflow venous vestibule-encircling ablations, which produced an AF suppression rate of 87% in a preliminary study published last year by Schwartzman et al (Journal of Interventional Cardiac Electrophysiology, October 2003, Vol. 9:2, pp. 295-300).
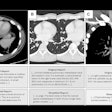
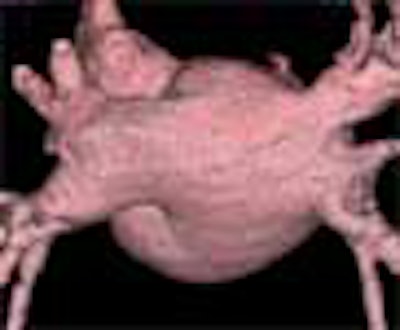
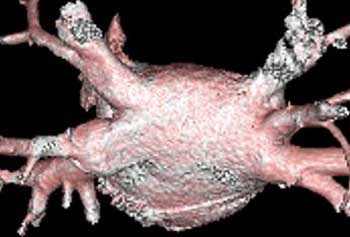
 |
| Above, volume-rendered MDCT epicardial view of atrium depicts four pulmonary veins: right superior (RS), right inferior (RI), left superior (LS), left inferior (LI). Image courtesy of Dr. Joan Lacomis. |
Complications
Notwithstanding the success of the procedure in a wide range of AF patients who do not respond to pharmacologic therapy, frequent minor complications, and occasional major ones, arise from the application of thermal energy to the thin-walled structures, Lacomis said.
Pulmonary vein stenosis, which can occur up to eight months after the procedure, remains the commonest complication, and unfortunately the symptoms are nonspecific: cough, shortness of breath, maybe hemoptysis. On chest films they can appear as patchy opacities in the lung that can mimic edema, focal areas of pneumonia, or even lung cancer, and they have been known to cause V/Q mismatches, she said. Endocardial charring, pulmonary vein dissection, and perforation can also occur.
"Bradyarrythmias are related to the fact that the vagas nerve fibers ennervate the distal myocardial sleeves," Lacomis said. "If you irritate them, the heart rate's going to drop."
Preprocedural MDCT
Electrophysiologists who perform RFCA have come to realize that success is less dependent on electrical mapping of the foci than on understanding the complex 3D anatomy of the target, usually the atriopulmonary venous junction, Lacomis said.
"In the past, much time during the procedure was wasted trying to fluoroscopically identify the target before the pulmonary veins could be mapped, and essentially before they could be ablated and the procedure even started," she said. "It has been shown that neither fluoroscopy nor transesophageal (echocardiography) are adequate for displaying this complex 3D anatomy. It is images such as this obtained with MDCT that have changed the way ablation therapy is done."
MDCT can easily identify the veins, document their number and location, their relationship to each other, the relationship of the pulmonary veins in the left atrium, and the size, shape, and volume of the left atrium. "We also try to exclude (the presence of) thrombi…it's extremely important because radiofrequency catheter ablation is contraindicated in people who have thrombi," Lacomis said.
The imaging technique is quite similar to CT angiography, but it might be better to think of it as a modified pulmonary embolism study, she said. "This is a kind of a hybrid study between looking at pulmonary veins, which belong to the lungs, and the left atrium, which belongs to the heart," she said.
Following an initial test bolus of 20 mL of contrast, a scanning bolus of about 125 mL of nonionic contrast is injected at 4 mL/sec with calculated timed delay or bolus tracking, which is recommended if there are wide variations in cardiac output.
For gated exams on a four-row scanner (GE Healthcare, Waukesha, WI), the group uses a 0.5-sec cardiac segment, 70% R-R interval, 1.25-mm collimation, and high-speed mode (6:1 pitch). The images are reconstructed to 1.25 mm using lung and soft-tissue algorithms, and postprocessed on a workstation (protocol originally reported in Radiographics, October 2003, Vol. 23, special issue, pp. S35-S50).
"To gate or not to gate, that is the question," Lacomis said. "If you gate you clearly get decreased motion artifact. We do pay a cost in radiation dose (and) typically gate patients …who have paroxysmal atrial fibrillation; they're not in afib(rillation) when they show up for the CT scan. So if they're in normal sinus rhythm, particularly if they're rate-controlled, or they're in a slow, stable rate of AF, we'll go ahead and try to gate them. You need gating if you're going to start looking at left atrial or left atrial appendage function. (But) a lot of our patients we do ungated. If they're too tachycardic to match the gating algorithm on your scanner, go ahead and do the non-gated." Contrast timing becomes more critical in non-gated images, she said.
Helping the electrophysiologist
What does the electrophysiologist need? "What our electrophysiologists really like to see are extra-atrial or so-called epicardial and endoatrial, endocardial, volume-rendered views -- so we can really show them the three-dimensional anatomy," Lacomis said. "But they don't want to see the whole heart. They want to see only the left atrium, the left atrial appendages, and the distal pulmonary vein. The segmentation algorithms available on the workstations are helping to exclude some of the other adjacent anatomy, and are quite helpful in time-saving."
Multiplanar reformats can be displayed in rotating 3-D views or filmed for reference during the RFCA procedure. The source images should be reviewed to identify anomalous veins, persistent left SVC, to clarify which normal veins drain which lobe or segment of the lung, to search for thrombi, and to exclude significant incidental findings. Lung windows on the workstation are often helpful for determining which lobe or section a pulmonary vein is draining, she said.
The source images are used to identify anomalous veins, identify persistent left SVC, clarify which normal veins drain which lobes or lung segments, search for thrombi, and exclude significant findings such as pulmonary fibrosis.
"We've had a couple of patients who presented with lung cancer," she said. "Have that treated before they undergo an ablation procedure."
Endocardial and epicardial views
Endocardial volume-rendered views of the right and left pulmonary inflow vestibules show the imaging volume from the intra-atrial perspective. They are useful for assessing global size, the shape and volume of the left atrium, the number of veins, accessory veins, and conjoined veins, and for examining the ostial branches and their intravenous saddles, the region of the atrial wall interposed between separate ipsilateral pulmonary veins.
"The electrophysiologists like it because it gives them kind of a global look at endocardial anatomy," she said.
Epicardial volume-rendered views should include the left atrium and the distal third of the pulmonary veins, but not the heart, pulmonary arteries, aorta, or vena cavae, in order to show the relevant structures without their being obscured by the adjacent anatomy.
"On the posterior (epicardial) view you can see clearly four pulmonary veins on this patient," she said. "We can look for ostial branches (defined as branches that join the main pulmonary trunk within 5 mm of the AV junction). And as we rotate this left atrium around, we really get a good feel for local LA size and shape."
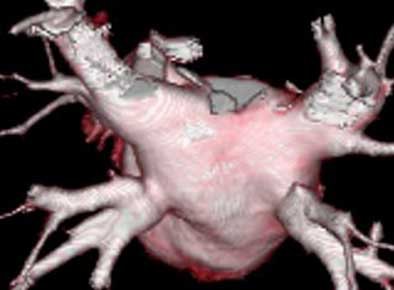
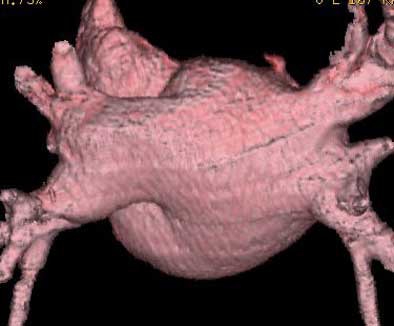
 |
| Endocardial (above) and epicardial (below) views from a patient with an accessory vein on the right (which happened to be vein that drained the right middle lobe (RML) and superior segment right-lower lobe (ssRLL), as well as separate LS and LI veins (left side seen in epicardial view only), for a total of five pulmonary veins. Images courtesy of Dr. Joan Lacomis. |
 |
Measurement
To aid in preprocedural planning, measurements can be taken of the LA volume, the PV or ostial diameters and circumferences, vessel segment length, saddle distances, and ostial noncircularity.
"When I first started doing it we spent a lot of time doing a lot of measurement," she said. "Now our doctors are really interested in me providing them left atrial volumes, again because we do posterior vestibule ablation." But for many patients, radiologists still need to take the time to measure the ostial diameters and circumferences, which must be done via orthogonal views on multiplanar reformats, she said.
"Just remember that these are not static structures," Lacomis said. "We're hoping to present some data at the RSNA this year showing how much these AV junctions change in diameter just in the course of a normal R/R interval."
Pulmonary vein anatomy varies widely, and right-side anatomy tends to be more complex than the left side, she said. At her institution, a third of patients have been found to have accessory veins, she said, usually on the right side (87%). Conjoined veins are less common (10%) and usually occurred on the left side (88%). About 70% of patients in the past five years have been reported to have the classic fourth pulmonary vein, and some form of variant PV anatomy is present in about 30%-40% of patients. Long-trunk common veins are easy to diagnose, short-trunk common veins more difficult, she said.
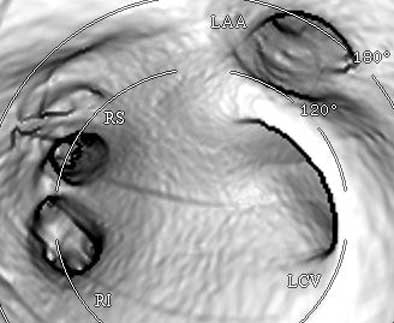 |
| Endocardial (above) and epicardial (below) views from a patient with a long-trunk left common vein (LCV) and separate RS and RI veins. There are a total of three pulmonary veins. (LAA = orifice of the left atrial appendage.) Images courtesy of Dr. Joan Lacomis. |
 |
On the horizon
Within the next few years, radiologists can look forward to real-time cardiac catheter navigation, as reported in an animal study by Solomon and colleagues, who used an electromagnetic catheter tip position-sensing system superimposed on 3D CT images (Journal of Interventional Cardiac Electrophysiology, February 2003, Vol. 8:1, pp. 27-36).
"Or can we go one step further?" she asked. "Can we at some point take 4D volume renderings of the left side of the heart…and fuse the electrophysiology data so it's more of a real-time thing? Can we take all of this and get a better view of the physiology of the left atrium and the mechanics of the left atrium?"
Radiofrequency catheterization is being used ever more commonly to treat atrial fibrillation, she said. Performing it successfully means not only curing afibrillation, but minimizing complications from the procedure.
"Left atrial and pulmonary venous anatomy is often complex -- much more complex than has been previously realized," Lacomis concluded. "Three-dimensional MDCT of the left atrium and pulmonary veins rapidly and reliably provides the necessary anatomic information for (RF) ablation catheterization success."
By Eric BarnesAuntMinnie.com staff writer
July 23, 2004
Related Reading
TE
echocardiography guides treatment of patients with atrial fibrillation, April 18, 2004
IBI ultrasound ablation efficient in atrial fibrillation procedure, July 18, 2001
Radiofrequency ablation safe and effective for atrial fibrillation, November 29, 2000
Copyright © 2004 AuntMinnie.com