Virtual colonoscopy computer-aided detection (CAD) systems are complicated beasts to study and compare, owing to their complexity, as well as the particular CT data that CAD algorithms are adapted for, or "trained" on in the parlance of their creators. CAD systems tend to work best on the population they're trained on, because abnormalities typical to the patient cohort being studied are defined for the system.
The U.S. National Institutes of Health (NIH) in Bethesda, MD, has developed one of the more sophisticated VC CAD systems to date, one that performed quite well in a large cohort of screening virtual colonoscopy patients, a third of whom were used to train the CAD system.
That study examined an asymptomatic cohort of 1,186 individuals over 50 years of age. The CAD's per-patient sensitivity for adenomas 8 mm or larger in the test set, 85.4% (41/48 [72.2%, 93.9%]), was comparable to that of optical colonoscopy prior to segmental unblinding, 89.6% (43/48 [77.3, 96.5]), according to the study results (Gastroenterology, December 2005, Vol. 129:6, pp. 1832-1844).
"We've shown CAD to be successful in a large-scale standalone trial," said Dr. Ronald Summers, from the NIH. "This particular CAD system was trained and tested on Department of Defense beneficiaries in San Diego and Washington, DC. One issue was whether this performance would extend to a civilian population in a different location."
The new study, which Summers presented at the 2006 RSNA meeting in Chicago, sought to evaluate the performance of an existing CAD system on virtual colonoscopy data from a new patient population.
Medical student Laurie Handwerker led the study, along with her colleagues at NIH, including Summers; Robert Van Uitert, Ph.D.; Keshav Deshpande; Srinath Yeshwant; Jack Yao, Ph.D.; and Marek Franaszek, Ph.D. Dr. Perry Pickhardt from the University of Wisconsin Medical Center in Madison provided the patient data.
The CAD system was trained on 374 of the 1,186 patients in the 2005 study, but left untrained for the new VC population. In an e-mail to AuntMinnie.com, Summers detailed how the CAD's classifier function is trained to scan the data for features likely to be polyps, using forward stepwise feature selection.
"The classifier we use is called a committee of support vector machines (SVM)," Summers explained. "We first run forward stepwise feature selection to reduce the large number of computed features (over a hundred) to a manageable few (about 15). Then we train the support vector machine committee, which is a set of SVM classifiers trained using a subset of the reduced set of features. The final committee consists of 70 support vector machines, in seven banks of 10 SVMs each. Each SVM uses three features but all the SVMs in a bank use the same three features (each SVM is trained on slightly different data using 10-fold cross-validation). Some of the features are used in more than one bank; that is why the final number of features is not 21."
The study examined average-risk patients who underwent same-day optical colonoscopy at the University of Wisconsin Hospital and Clinics in Madison following CT colonography (CTC or virtual colonoscopy [VC]).
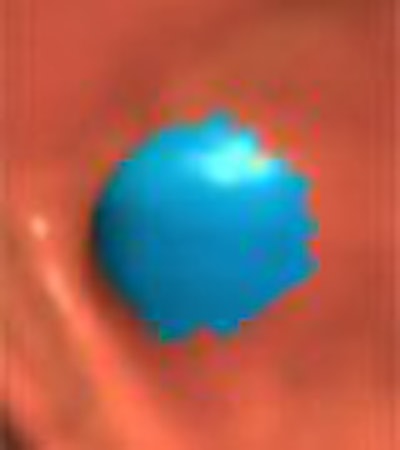
 |
| The CAD system's multistage analysis process includes colon segmentation with electronic fluid subtraction, surface shape analysis, segmentation of polyp candidates, and finally, classification. All images and data courtesy of Dr. Ronald Summers. |
"The population consisted of 170 patients at average risk of colorectal cancer," he said. "They had same-day optical colonoscopy only if CTC was positive. This was a special set of patients at the University of Wisconsin that had polyps 10 mm or larger. Those with polyps 6-9 mm go on to (colonoscopy and) polypectomy, only they do not wish to be in a surveillance population and undergo CTC within one to two years' time."
Bowel prep included a clear liquid diet, 10 mg of bisacodyl, 45 mL of sodium phosphate, 250 mL of 2.1% barium, and 60 mL of diatrizoate meglumine, a water-soluble iodinated oral contrast agent.
The subjects were scanned with either room air or automated CO2 (ProtoCO2l, E-Z-EM, Lake Success, NY) insufflation. Supine and prone images were acquired on either an eight- or 16-detector scanner (GE Healthcare, Chalfont St. Giles, U.K.), reconstructed using 1.25-mm thick slices with a 1-mm reconstruction interval, and 50-75 effective mAs.
The cohort had 85 colonoscopy-confirmed adenomas, including 46 that were 10 mm or larger in 40 patients, 39 6- to 9-mm adenomas in 34 patients, and two cancers, Summers said. Overall sensitivity was comparable to the previous trial results.
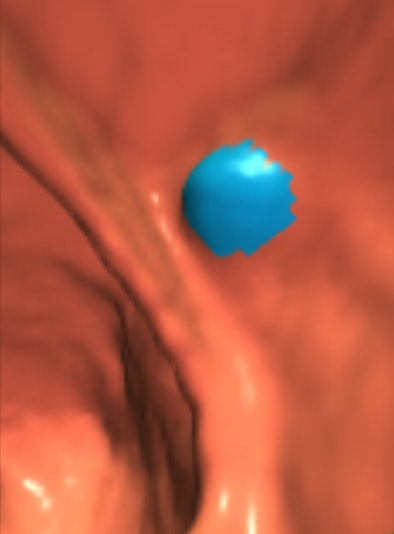 |
| A 9-mm adenoma detected by CAD. The image was produced using V3D Colon software (Viatronix, Stony Brook, NY). The blue coloring is the CAD detection on the polyp. |
"The sensitivity per adenoma in this study, compared to that of our original (Department of Defense) study, for large polyps was 87.0% (40/46) for this study and 92.9% (26/28) for DOD," he said. For medium-sized polyps, sensitivity was 82.1% (32/39) versus 49.5% (45/91) in the DOD study, and this difference was statistically significant."
Per-patient sensitivities in the DOD study were 60.3% (44/73) and 92.9% (26/28) for 6- to 9-mm polyps and those 10 mm or larger, respectively, and 82.4% (28/34) and 92.5%, respectively, in the current study. Mean and median false-positive rates were 6.7 ± 6.4 and 5.0, respectively in the DOD study, and 8.4 ± 7.9 and 6.5, respectively, in the present study.
"There were two cancers and CAD detected both of them, including a multilobulated cancer and napkin ring or annular cancer," Summers said.
In all, there were 25 false negatives, including those on or adjacent to haustral folds (48%), on the air-fluid boundary (8%), and in collapsed colonic segments (8%). "In addition, we observed that almost two-thirds of the false negatives had adherent contrast, which is a phenomenon where oral contrast sticks to polyps for reasons that are unclear," Summers said.
The false positives were due to thick haustral folds (35%), residual stool (30%), the rectal tube in 15%, and the ileocecal valve in 10%.
The study limitations included colonoscopy referral only in patients who were positive at VC, "although I believe this patient population will become one of greater interest in the future if we move to a paradigm where smaller polyps are observed rather than removed," Summers said. "We would not have optical colonoscopy follow-up in these cases."
Also, only CAD's standalone performance was evaluated, and not its use by radiologists, he said.
"This system had a high level of performance on a new patient population," Summers said. Its sensitivity for large polyps, false-positive rate, and reasons for false negatives and positives were similar to those of our previous study, and based on these results, we conclude that our CAD system is generalizable to a new patient population."
By Eric Barnes
AuntMinnie.com staff writer
February 5, 2007
Related Reading
VC CAD face-off reveals system differences, November 27, 2006
CAD will be key to VC screening, September 13, 2006
VC CAD helps junior readers catch up, May 4, 2006
VC visualization tool combines advanced features, August 17, 2006
Telltale barium coating could help VC find risky polyps, March 15, 2006
Copyright © 2007 AuntMinnie.com




















