One size does not fit all in the realm of renal artery stents. A review of abdominal multidetector-row CT (MDCT) angiograms found significant size differences between left and right vessels, between men and women, and between patients.
These differences have serious implications for the increasingly common use of distal filtering devices to prevent distal atheroembolization after percutaneous transluminal renal angioplasty (PTRA). The devices are designed for carotid and coronary arteries, and more often than not don't fit, the authors report (American Journal of Roentgenology, June 2007, Vol. 188:6, pp. 1652-1658).
Size matters mightily in the setting of PTRA, which is becoming first-line therapy for ostial atherosclerotic renal artery stenosis. The procedure is popular because it is seen as a safe, reliable, and cost-effective method of treating many patients with refractory hypertension and chronic renal insufficiency.
But along with concerns about patient selection and contrast-induced nephropathy, the risk of distal atheroembolization looms large, and "is considered to play a key role in limiting the quality of outcome after PTRA, indicating the need for distal embolic protection during the procedure," wrote Dr. Adam Talenfeld, Dr. Ryan Schwope, and their colleagues at Mount Sinai Medical Center in New York City.
But there is no dedicated protection device designed to catch renal artery clots, according to the authors, so interventionalists have turned in large numbers to the off-label use of carotid or coronary filters.
"Early enthusiasm for this practice has been tempered, however, by concerns about the inadequate design of these devices for use in the renal circulation," the authors wrote. "To our knowledge, our study was the first performed with 3D imaging of the renal artery anatomy as pertains to PTRA with distal protection."
Using such filters in the renal arteries means overcoming the challenges associated with the anatomy, however. The study authors used 3D image reconstruction to review high-resolution MDCT angiography data to characterize the anatomy of stenotic renal arteries.
The group examined 218 abdominal MDCT angiograms from a tertiary care center. All images were acquired with 16- or 64-detector-row scanners. Data were acquired at 16 x 0.75 mm for the 16-slice scanner, and at 64 x 0.6 mm for 64-slice CT. "Cross-referenced axial, coronal, and sagittal projections were used to select the renal arteries from a volumetric dataset," the authors noted.
Among the 108 patients (32 women, ages 57-92, mean age 79; 76 men, ages 58-97, mean age 77) were 127 native renal arteries (72 left, 55 right) with more than 50% ostial atherosclerotic renal artery stenosis. Accessory renal arteries and those with stents already in place were excluded from the analysis.
 |
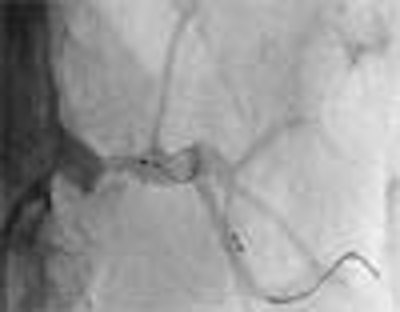
| A 71-year-old man with malignant hypertension and renal insufficiency. Angiogram shows inadequate diameter of basket and excessive length of wire distal to basket. Image republished with permission of the American Roentgen Ray Society, from AJR 2007; 188:1652-1658 by Adam D. Talenfeld, Ryan B. Schwope, Huntley J. Alper, Emil I. Cohen, and Robert A. Lookstein. |
The authors used 3D vascular reconstruction software (Vitrea 2, Vital Images, Minnetonka, MN) to review renal MDCT angiography data retrospectively, quantifying the "anatomic limitations" involved in placing distal protection devices for the management of ostial renal artery stenosis. They measured renal artery length, cross-sectional area, and maximum diameter.
A two-tailed Student's t-test was used to measure the differences between mean values for women and men, and for left and right renal arteries. To compensate for potential error, multivariate regression analysis was used to determine the strength of the relationship between gender and anatomic dimensions, and the relationship between renal artery anatomy and subject age.
"The length of each renal artery was measured in two segments: L1, defined as the distance from the ostium to the point of maximum stenosis; and L2, defined as the length from the point of maximum stenosis to the bifurcation of the main renal artery," the authors explained. "The sum of these two values was defined as the effective length of the main renal artery."
The results showed significant differences for men and women in the average maximum cross-sectional area distal to the point of stenosis (0.3 ± 0.19 versus 0.23 ± 0.09 cm², p = 0.006) and the corresponding maximum diameter (6.9 ± 1.7 versus 6.1 ± 1.1 cm², p = 0.003), the authors wrote. The average lengths of the main renal artery did not differ significantly for men and women.
The left renal arteries were an average of 3.9 ± 1.3 cm long; the right renal arteries a cm longer at 4.9 ± 1.6 cm, the group reported. Concordant lengths and differences were seen in the distal protection device landing zone or the length of the main renal artery beyond the point of maximum stenosis.
Vessel diameter also varied significantly. The mean maximum diameter from the point of maximum dilatation to the point of stenosis was close to 7 mm (6.9 ± 1.5 mm) in the left renal artery and 6.5 mm (6.4 ± 1.6 mm) in the right renal artery, the group reported. Among men, the mean left point of maximum dilatation was almost 1 mm larger than the mean right (7.2 ± 1.6 versus 6.4 ± 1.8 mm).
The size differences have important implications for the off-label use of distal protection devices, according to the authors.
"Our findings suggest that most commercially available distal protection devices do not accommodate renal artery anatomy for PTRA," they wrote. "For example, the GuardWire (Medtronic, Minneapolis) balloon occlusion and aspiration system is short enough in the main renal artery landing zone for that device to appear ideal to protect most renal arteries, even those with a short distance between the aorta and the renal artery bifurcation. However, the balloons of currently available GuardWire models inflate only to a diameter of 3-6 mm, in many cases leaving emboli considerable room to pass."
In addition, they explained, most protection devices approved for use in coronary and carotid interventions filter only up to 6 mm of the vessel diameter, and typically occupy a large portion of the length of the main renal artery.
"In our study, the average distal protection device landing zone ... was 3 cm in most of the vessels, a length that would not accommodate most devices on the market. Our results did show that the crossing profiles of most currently available distal protection devices (3.2-3.5 French, or 0.0089-0.0107 cm²) should accommodate most atherosclerotic lesions (mean area at maximum stenosis for all subjects, 0.07 ± 0.04 cm²), the authors wrote.
The results suggest that simple design modifications may improve the safety and efficacy of distal protection devices in renal circulations, and improve outcomes afterward, they concluded. "Because of the variability of anatomic features among individual patients, our results also suggest the utility of 3D imaging analysis of all patients before they undergo PTRA," they added.
By Eric Barnes
AuntMinnie.com staff writer
June 13, 2007
Image-guided biopsy may help avoid nephrectomy, May 4, 2006
Percutaneous cryoablation helpful against renal tumors, March 8, 2006
Angioplasty of ostial renal artery stenoses lasts longer with stenting, August 18, 2000
DSA readings vary widely in estimating renal artery stenosis, November 11, 1999
Copyright © 2007 AuntMinnie.com