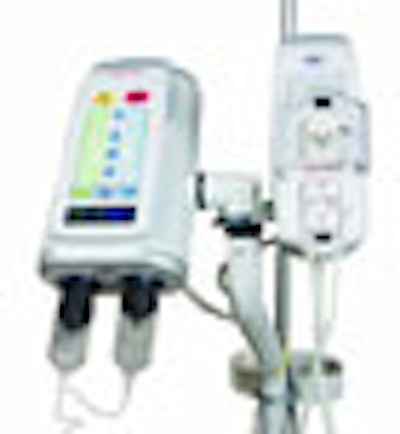
(Booth 8513) Medrad of Warrendale, PA, is set to roll out its new CardiacFlow upgrade for the company's Stellant D CT contrast injection system. Designed to compute customized contrast injection protocols for each cardiac patient, this enhancement permits cardiac imaging during ECG gating.

In addition, Medrad will introduce a new patented safety feature for Stellant D, called the XDS extravasation detector technology. XDS couples radiofrequency waves with a physical property called permittivity that directly detects fluid pooling under the patient's skin. Once the system senses mild extravasation, it stops the injection to reduce the risk of a mild occurrence becoming moderate or severe.
XDS received clearance from the FDA in March 2007, and is commercially available.