A new computer-aided detection (CAD) scheme that maps haustral folds in the colon could benefit virtual colonoscopy (VC or CT colonography) interpretation in several important ways.
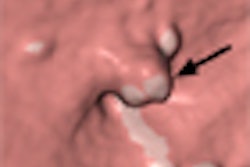
The human colon has many haustral folds, which complicate interpretation of the long, convoluted anatomy with virtual as well as optical colonoscopy. As a result, radiologists reading VC data must frequently change the viewing angle to examine the colon in 3D endoscopic views -- and still risk missing polyps and cancers that are hidden in the folds.
CAD schemes are similarly confounded by haustral folds. Current automated methods that detect polyps based on convex shapes are often fooled by the ridgelike structures lining the colon wall, leading to false-positive detections of polyps that turn out to be haustral folds. At the same time, true polyps located on or near the folds are easily missed by CAD algorithms and radiologists alike.
If haustral fold detection and analysis could be performed before polyp detection, say researchers from Japan, it would be much easier to determine if a particular feature in the CT data were a polyp or a fold, a polyp on a fold, or a plain old polyp. And it might be easier to find flat polyps, which alter the colonic mucosa in subtle ways that aren't always visible to the naked eye or to typical CAD algorithms.
"Previous CAD methods detected convex-shaped parts of the colon, so flat-shaped polyps are missed," said principal investigator Masahiro Oda from the Graduate School of Information Science at Nagoya University in Nagoya, Japan. "Because the thickness of haustral folds increases when flat-shaped polyps are located on them," flat-polyp detection could be improved by first detecting polyp folds to reveal any abnormal shapes and thicknesses, Oda said at the 2008 Computer Assisted Radiology and Surgery (CARS) meeting in Barcelona, Spain.
CT images in supine and prone positions are also used for diagnosis to increase sensitivity of polyp detection, and it is often difficult and time-consuming to match the two datasets, a process used to match lesions, assess their location, and distinguish polyp from stool. Synchronized display of two CT datasets might speed up this task as well.
"Haustral fold detection could also be used for correspondence of prone and supine datasets, Oda said. "CT images in supine and prone positions are used for diagnosis to increase sensitivity of polyp detection. Synchronized display of [prone and supine] CT datasets will improve efficiency of diagnosis, because the positions of haustral folds are utilizable as landmarks for registration."
The Nagoya researchers, along with colleagues from the University of Tokushima in Tokushima, Sapporo Minami Hospital in Sapporo, and other institutions, developed a haustral fold detection algorithm that performs better than an earlier method, although additional features will be needed before the new method can be used to answer important clinical questions, Oda said.
Depicting haustral folds
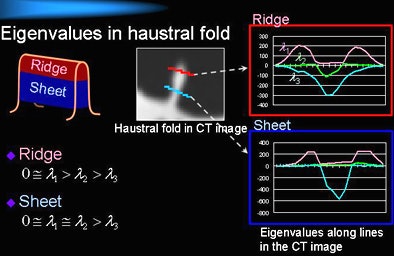
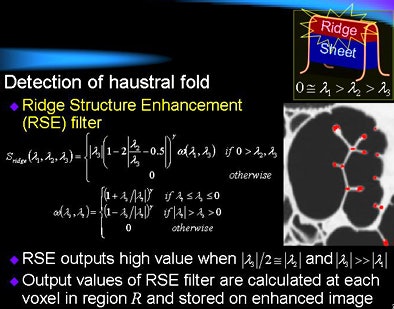
The haustral fold detection method employs a ridge structure enhancement filter based on eigenvalues of a Hessian matrix, Oda explained. First, it assesses the approximate distribution of CT values in the image data by fitting a quadratic polynomial equation at three data points for each voxel, each representing a local cubic region.
A Hessian matrix is then calculated using second-order partial differential coefficients of the fitted surface. The output of a ridge structure enhancement (RSE) filter is then calculated from the eigenvalues of each voxel, and the output is used to generate an enhanced image of haustral fold regions.
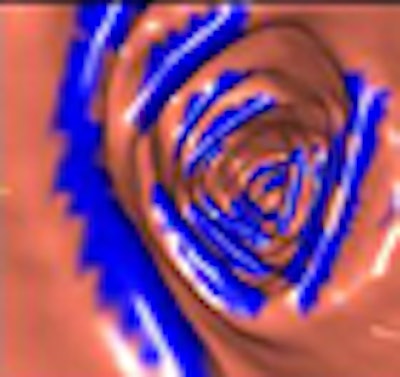
 |
| The proposed haustral fold detection method employs an RSE filter calculated from the eigenvalues of each voxel, using the output to generate an enhanced image of the haustral fold regions. All images courtesy of Masahiro Oda. |
 |
False positives are reduced by eliminating the smallest voxels, eliminating the smallest connected regions, and eliminating the connected regions with the lowest connectedness values, Oda said. The remaining output is then classified into haustral fold regions.
Once the folds are detected in both prone and supine data, the folds are numbered and used to manually generate supine and prone correspondence of VC data, he said.
Oda and his team applied their method to MDCT virtual colonoscopy data from 12 patients. Three-dimensional CT datasets, acquired using a 512 x 512-pixel image matrix, collimation of 1.25 mm, and slice thickness of 0.6-0.7 mm, were analyzed for the study.
Except for the smallest structures, nearly all of the haustral folds were detected, "including 81.7% (1,326/1,624) of haustral folds, with 6.9 false positives per case," Oda said. "Sensitivity was higher than a previous method," he said.
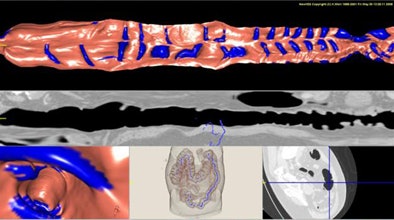 |
| The results show detected haustral folds marked in blue. |
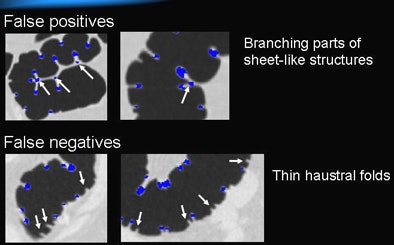 |
| Most false positives were generated by small branches of sheetlike structures. Most false negatives resulted from thin haustral folds. |
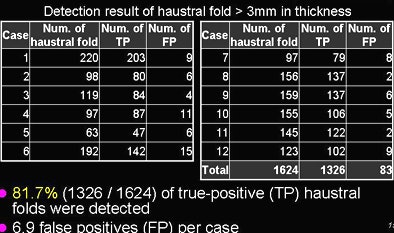 |
| Results table shows the number of haustral folds, true positives (TP), and false positives (FP) (in each of 12 cases). Sensitivity was 81.7% with 6.9 false positives per case. |
In the previous study, published in 2000, Sato and colleagues proposed a sheet structure enhancement filter. The method succeeded in detecting haustral folds with a sensitivity of 76.3%, but it led to overdetection of haustral folds in areas of CT images that were sandwiched between colonic wall regions, Oda said of the study (IEEE Transactions on Visualization and Computer Graphics, April-June 2000, Vol. 6:2, pp. 160-180).
The present study, using an RSE enhancement filter, succeeded in detecting the ridges of the haustral folds with greater sensitivity than the previous study. However, further refinements will be needed to take full advantage of the data generated, Oda said.
One drawback of the current method, for example, is that thin areas of the colon wall between two different parts of the colonic lumen are overdetected, he said. Because local intensity structures of these thin-walled areas are similar to those of haustral folds, these regions need to be removed from the detection to eliminate most of the remaining false positives, he said.
"Analysis of shape, size, and thickness of entire haustral folds is needed for flat-polyp detection," Oda said. "Detection method of entire haustral fold [structure] is needed."
"Future work includes improving accuracy of false-positive reduction process, further experiments using a larger number of CT images, and development of [automated] supine-prone registration and lesion detection methods using haustral fold information," he said.
Once a polyp detection method has been developed to utilize the data from haustral fold analysis, detection of flat polyps is expected to improve via the measurement of haustral folds. The presence of subtle local increases in haustral fold thickness can be used to improve the detection of flat polyps in CAD schemes, Oda said in response to a question from the audience.
By Eric Barnes
AuntMinnie.com staff writer
September 12, 2008
Related Reading
All-purpose CAD tool stretches imaging boundaries, July 7, 2008
Teniae coli landmarks guide navigation in VC, January 2, 2008
VC measurements more accurate than optical colonoscopy, May 30, 2007
New method corrects for hyperattenuation surrounding tagged VC data, August 8, 2006
Colon muscles do heavy lifting in polyp positioning, October 19, 2005
Copyright © 2008 AuntMinnie.com