An injected monoclonal antibody is offering hope of preventing gastrointestinal syndrome in patients exposed to high levels of ionizing radiation. A new study in the Journal of Clinical Investigation found that administration of the anticeramide antibody prevented cell death in blood vessels of the gastrointestinal (GI) tract and improved 90-day survival among mice exposed to 15 Gy of whole-body irradiation.
GI syndrome involves destruction of crypt-villus units, loss of mucosal integrity, and infection by resident enterobacterial flora, wrote Jimmy Rotolo, Dr. Richard Kolesnick, and colleagues at Sloan-Kettering Institute in New York City (JCI, April 2, 2012). Symptoms include anorexia, vomiting, diarrhea, dehydration, systemic infection, and, in extreme cases, septic shock and death.
Traditionally, unrepaired or misrepaired DNA double-strand breaks in stem cell clonogens are thought to produce irreversible tissue injury, but recent studies offer evidence that acute endothelial damage also plays a major role in GI tract injury, the researchers said. The damage occurs when exposure to high-dose radiation kills the specialized stem cells found in the epithelial lining of the small intestines and colon, destroying the protective epithelial barrier, or mucosa, and leading to the onset of radiation GI syndrome within days of exposure.
In the current study, the researchers wanted to identify a prophylactic agent that might prevent this kind of tissue damage. They examined the effect of administering monoclonal antibody 2A2 before the radiation was applied to prevent apoptosis in the intestines of mice.
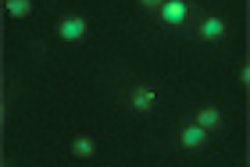
The authors identified endothelial apoptosis via microscopic detection of TUNEL (brown) and CD34 (red) double-positive endothelium. Mouse survival postradiation was calculated using the Kaplan-Meier method, and GI damage was diagnosed as the cause of death when small intestines displayed denuded mucosa with nearly no villi or crypts, or when mucosa displayed limited repair.
According to the results, pretreatment with monoclonal 2A2 antibody injection reduced ceramide-rich platforms to 16% ± 2% of the study population, which was not significantly different from the baseline 15% ± 3% found in unirradiated cells (p > 0.1), the authors reported. Thus, administration of the antibody inhibited endothelial apoptosis in blood vessels within the GI tract and facilitated recovery of crypt SCCs, improving 90-day survival in the mice from 0 to 80% in mice exposed to 15 Gy whole-body irradiation.
"We suggest that 2A2 represents a prototype of a new class of anticeramide therapeutics and an effective countermeasure against radiation GI syndrome mortality," Rotolo and colleagues wrote.