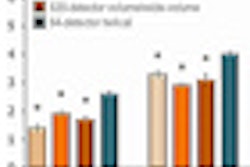
SAN FRANCISCO - An evolving CT dose-reduction technique looks beyond simulated image noise as a measure of image quality, and instead aims to optimize dose based on what the radiologist needs to see, according to a presentation on Monday at the International Society for Computed Tomography (ISCT) annual meeting.
Developed by researchers from the University of Maryland in cooperation with Philips Healthcare, the work-in-progress application is designed to more accurately depict the noise that results from low-dose scans, said presenter Dr. Eliot Siegel. The better representation will then be applied to quantitative perception criteria to optimize dose.
In the future, image quality assessment via the visual perception system will be used in combination with more realistic and quantitative representations of image noise than are possible in current systems, according to the researchers. The goal, they say, is to be able to use sophisticated mathematical models to reduce dose automatically for every exam to levels that minimize dose without impairing the quality of the image as the reader sees it.
 Dr. Eliot Siegel, from the University of Maryland, spoke at the ISCT meeting.
Dr. Eliot Siegel, from the University of Maryland, spoke at the ISCT meeting.
Traditionally, the term image quality has been difficult to define. Diagnostic image quality is at least understandable, while "good" and "optimal" image quality are not.
In fact, it's entirely subjective, but that reality is changing with advances in quantitative image analysis, Siegel said.
As awareness of radiation dose continues to increase going forward, image acquisition and quality are going to be assessed in more objective and quantitative ways than in the past, Siegel said. As part of that evolution, simplistic methods of simulating low-dose images -- for example, by simply adding noise as today's low-dose software does -- are on the way out.
Today's Poisson models aimed at simulating the noise that accompanies low-dose scanning do not work well with iterative reconstruction schemes, which often keep noise constant as the radiation dose drops.
More important, Poisson models lack the ability to mimic noise from the detector itself; this shortcoming plays a major role in image degradation at very low dose levels. "There are lots of things that happen at really low doses that create modifications to the image that are not simulated with just low noise," Siegel said.
But for all the effort spent on dose reduction, little has been written about establishing a methodology to optimize dose based on more advanced criteria, he said.
Reader perception is key to building a better methodology, Siegel noted. As the radiation dose keeps dropping and a CT image keeps changing and getting noisier, at what point does the reader notice that the image has changed? At what point will he or she miss the diagnosis?
Adjusting the dose based on what the radiologist is looking for is a well-known concept and an important one that the project hopes to optimize.
"From the research we've done in many different areas, what looks aesthetically pleasing does not always correlate with diagnostic efficacy," which is variable, he said. Reader perception studies are time-consuming, tedious, and subjective.
Distortion metrics
In a different approach, the field of visual discrimination model (VDM) distortion metrics uses a mathematical model that simulates physiological responses of the eye and visual cortex to patterns of contrast, Siegel said. Traditionally used for studies of image compression, it allows researchers to determine to what extent a human can make the distinction between one image and another image.
Much research using VDM has gone into image compression studies, which aim to determine how much data compression can be applied before an image is perceived as different from a full-size dataset. The concept has not been applied to dose reduction, but it has much more potential than signal-to-noise ratio to determine what makes a diagnostic image, "comparing a higher-dose with a lower-dose image and then asking the question, 'Is there a perceptual difference?' " Siegel said.
The point at which the change is noticed can be expressed as JND, or "just-noticeable difference," which can be scored numerically to grade the degree of difference between two images, he explained.
JND data can be used to determine optimal dose and could help determine the impact of dose-reduction algorithms or alternative reconstruction techniques such as iterative reconstruction. The results could be used to determine optimal dose for a repeat CT study or could determine optimal dose on the fly while a study is being performed.
The application being developed by Siegel and colleagues includes a mixture of pink and white noise corresponding to the Philips scanner output, as well as image artifacts. The images are very realistic, and the results of a simulated 10-mAs scan are much closer -- both visually and quantitatively -- to a real scan acquired at 10 mAs, Siegel said.
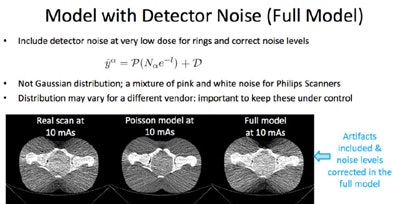 |
| Full model noise estimation of an image acquired in a pelvic phantom includes detector noise and artifacts. The result is virtually indistinguishable from a real ultralow-dose scan. All images courtesy of Dr. Eliot Siegel. |
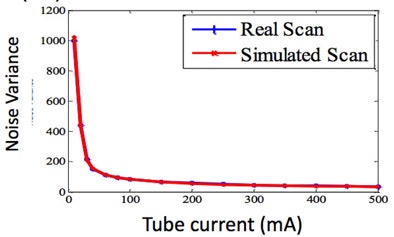 |
"When you take into account the detector noise, you have almost a perfect match between a real scan and a simulated scan," he said. "If we can simulate these really low doses, then we can start looking at the potential for taking a scan, simulating what it would be like at a lower dose, and then being able to determine what dose to actually use in our patients."
Accurate simulation of dose could serve in multiple ways, he said. Exams could be retrospectively reviewed and techniques could be set based on subjective image quality. And previous imaging studies could serve as a guide for using dose in a current patient visit.
"This optimization could be automated using this JND perceptual model," he said. "We could actually scan at lower doses by predicting the fact that there will be minimal difference" in the resulting images.
Once the JND is established, a scan could be acquired at a reduced dose based on a combination of JND and the simulated scan, Siegel said.
"The potential to add in detector noise to simulate lower doses gives us tremendous ability to be able to simulate what the actual image will look like," he said.
Siegel envisions a fully automated system that suggests a dose based on all of the available data. The radiologist could accept the automated suggestion, or simply use the tool manually to try out potential dose settings until they deliver the elements the radiologist wants to visualize.