How big should a lung nodule be before it can be considered worthy of diagnostic workup? It's not just an academic question, as it plays into the growing debate over the clinical value and cost-effectiveness of CT lung cancer screening, according to a study published online February 18 in the Annals of Internal Medicine.
Boosting the nodule size threshold for a positive CT lung cancer screen from the current 5 mm in diameter to perhaps 7 to 9 mm would significantly reduce the number and the costs and risks of workups for positive findings. However, the option needs to be weighed carefully, as it would require a trade-off involving delayed diagnosis of some cancers.
"As large national programs screen millions of high-risk participants, the definition of a positive result will have enormous cost implications," wrote a research group led by Dr. Claudia Henschke, PhD, from Mount Sinai School of Medicine, along with colleagues Rowena Yip, Dr. David Yankelevitz, and Dr. James P. Smith (Ann Intern Med, February 18, 2013).
One-fifth need workups
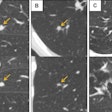
In a typical CT lung cancer screening cohort, as many as one-fifth of participants might receive positive results following detection of a nodule 5 mm or larger that could potentially represent lung cancer. But 5-mm nodules are rarely malignant.
Boosting the cutoff to 7 mm or 9 mm, for example, would bring the proportion of positive findings down to levels commensurate with other screening tests.
In the study cohort of more than 21,000 screening subjects, the group found that increasing the threshold for a positive result from 5 to 9 mm would reduce the prevalence of positive screens from 16% to 4%. On the downside, cancer diagnoses could be delayed for as long as nine months, raising the risk that a cancer might be missed in its earliest and most easily curable stages.
Previous studies have shown that the vast majority of noncalcified nodules smaller than 5 mm are either benign or are too small to identify whether they are growing at a malignant rate. What's more, they are too small to follow up with diagnostic tests or biopsy with current diagnostic tools until at least a year later, when an increase in size can be used to perform additional tests to get a better idea of the malignant potential of the nodule, explained Henschke.
And considering the small number of positive results in such diminutive nodules, the current 5-mm diameter cutoff isn't necessarily the optimal way to proceed, she told AuntMinnie.com.
"When we first started screening in 1993, we worked up everybody because we had no knowledge base," she said. By the time the first results were published a few years later, "we came up with the idea of making the cutoff 5 mm."
The 5-mm cutoff became the basis for the Fleischner guidelines and other international recommendations for managing small nodules. But some 10 years later, Henschke believes that the criteria might warrant a second look.
"CT scanners have advanced, our knowledge has advanced, and perhaps we should come up with some other criteria," she said.
Therefore, the research team aimed to assess the frequency of positive results as well as the potential delays in diagnosis in the baseline round of screening by using more restrictive thresholds for a positive exam. The study was based on an analysis of screening results in 21,136 participants of the International Early Lung Cancer Action Project (I-ELCAP) who were screened between 2006 and 2010.
Using the database, the researchers assessed the frequency of solid and part-solid pulmonary nodules and the rate of lung cancer diagnosis by using the conventional 5-mm size threshold, as well as alternative threshold values of 6 mm, 7 mm, 8 mm, and 9 mm.
Larger thresholds yield fewer positives
The authors found, not surprisingly, that the number of lesions considered to be positive declined as the size threshold went up in the study population.
Positive detections and delayed diagnoses by lesion size
|
As a result of the change, fewer patients would have been diagnosed with lung cancer during the first 12 months of baseline screening, decreasing the number of diagnoses by six participants using a 7-mm cutoff, seven participants using an 8-mm cutoff, and eight participants using a 9-mm cutoff. In the cohort used for analysis, however, all of the cancers with delayed diagnosis due to larger thresholds would still have been diagnosed at stage I.
In all eight cases of lung cancer that were actually diagnosed, the investigators logged nodule growth before the invasive diagnostic procedures were performed. All cancers were in clinical stage I before resection and were found to be in pathologic stage I after resection. There were no complications in any surgical biopsies or resections.
Size isn't everything
What constitutes a positive lung cancer screen should be continually reappraised to minimize the potential harms of further unnecessary diagnostic workup and costs while maximizing the frequency of early diagnosis, the group wrote.
No matter what the threshold, however, relying on nodule size alone to define a positive result is a study limitation considering that other nodule characteristics may distinguish cancer from nonmalignant nodules, Henschke and colleagues wrote. As investigators gain more experience, new distinguishing features of lung cancer will probably emerge, potentially from new image processing techniques, and these will improve the definitions and reduce the frequency of a positive result.
In an editorial accompanying the results, Dr. Stephen Lam, from the British Columbia Cancer Agency, and colleagues stated that relying on size alone is indeed insufficient -- as evidenced by the differing rates of malignancy in the cohort for solid versus part-solid nodules.
"We believe that a comprehensive computer-based risk calculator (prediction model) that integrates demographic characteristics and multiple CT image features (nodule size, type, and spiculation and emphysema status) would help to estimate risk for lung cancer for different types of lung nodules to guide clinical decisions on the basis of cancer probability," Lam and colleagues wrote. "To be accurate, such models must incorporate more information and provide probabilities of lung cancer over a range of nodule sizes, not just for one cut point."
Henschke agreed.
"We have been looking at algorithms all along; as a statistician that's what I would want to find," she said. "But size is really still the critical component; we haven't found very much that adds to the size criteria, and it's simple enough."
As for other limitations, the small number of cancer diagnoses that were delayed in the retrospective analysis prevents a fuller assessment of the harms of delaying the diagnostic process. Prospective assessment of any new size threshold should be undertaken to evaluate it fully, Henschke said.
"Clearly we would like to implement -- and we would hope that different people would implement -- different cutoffs, and we would collaboratively evaluate the pros and cons," she said. "And that could be done fairly quickly. After all, you know within one year how many late-stage cancers you find, or you've missed, so you could do a very effective prospective study very quickly. And I hope this [analysis] encourages people to do so."
In evaluating different thresholds prospectively, Henschke said her group will have meetings every six months to evaluate the impact, and she hopes they will develop consensus with other groups evaluating different standards. There are plenty of data left to evaluate for new size thresholds too; for example, all the data from the National Lung Screening Trial.
The key message of the study is that the definition of a positive CT lung cancer screening result needs to be continually evaluated and updated in light of emerging evidence from screening programs and advancing technology, in an effort to reduce unnecessary surgery for nonmalignant nodules and reduce the potential harms of diagnostic workup, Henschke and colleagues wrote.
"Everybody says you have all these false positives and, therefore, screening would be so expensive," she said. "But it doesn't have to be because the critical round is that first baseline round. If you can cut down on unnecessary follow-up for that round, then it really becomes quite efficient in subsequent rounds because you have the prior images to compare them with."
"It's really the baseline round that is expensive, and I think that cutting down baseline [workups] by 65% should be an incentive to perhaps provide more screening for people," she said. "But I'm not into policy -- I just provide the data for people to make those recommendations."