Toshiba America Medical Systems has received clearance from the U.S. Food and Drug Administration (FDA) for a myocardial perfusion feature for CT studies.
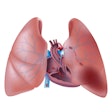
Available on Toshiba's Aquilion One and Aquilion One Vision CT systems, the myocardial perfusion feature is designed for visualizing myocardial ischemia, the firm said.
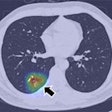
The software on 320-detector-row CT scanners shows blood flow and anatomy within the coronary arteries to help determine the viability of the heart muscle. This could help clinicians decide whether patients need to undergo revascularization of coronary blockages.
The software is also designed to provide shorter exam times and reduce radiation exposure, according to Toshiba.