You've heard of dual-energy CT, but how about eight-energy CT? That's the goal of researchers in New Zealand, who this month received funding for a project to build a CT scanner capable of detecting eight different energies at once in scans of humans. They hope the new spectral scanner will offer functional imaging that is unprecedented for CT, with a lower radiation dose to boot.
Spectral imaging is already being used in a few institutions, but commercially available scanners are limited to two energies. The new multienergy CT scanner is expected to go further, delivering eight separate energy spectra in every scan, and with it troves of data about disease states and changes to tissue produced by pharmaceuticals.
The project, which is funded mostly by the New Zealand government with help from corporate and academic sources, hopes to enable the precise measurement of different components of human tissues, according to project leader Dr. Anthony Butler, PhD, director of bioengineering at the University of Otago's Christchurch campus.
 Dr. Anthony Butler, PhD, from the University of Otago.
Dr. Anthony Butler, PhD, from the University of Otago."I think the most exciting part of this technology is that it takes CT from being an anatomical modality, where you just get the shape of an object, into a functional modality more like MRI or PET ... a molecular modality," Butler told AuntMinnie.com. Along with tissue components, "we can see pharmaceuticals in the tissue, and that allows us to do the sort of stuff you traditionally do on PET," he said. "Now we may not be as sensitive as PET, but we're a whole lot faster. We've got high spatial resolution, and we can do multiple pharmaceuticals at once."
Multienergy imaging
Multienergy imaging works by measuring the relationship between CT attenuation and photon energy in elements, using a photon-counting detector to discern the energy of incident x-rays and allocate them to separate bins. With this spectral power scaled up from micro-CT to human scale, the team envisions scanning multiple tissue targets dosed with multiple pharmaceutical agents in a single scan to reveal exquisite detail about human disease processes and how different drugs can affect them.
"The actual selection of energies varies with the concentrations of materials you're looking at and the body parts you're imaging, so you would use different energy settings between, say, the chest and abdomen," Butler said. "By having eight energy buttons, you can optimize the scan for all of the body parts at once."
Software helps the scanner operator choose the eight energies for each scan, which depend on the imaging target and are customizable from 15 keV up to 120 keV, Butler said. High energies can also be used to get rid of beam-hardening artifacts, and for all scans, the overall radiation dose to the patient will be cut significantly -- somewhere between 5% and 30%. Radiation dose could be driven down even further by the time iterative reconstruction processing is added, he said.
"You can use pharmaceuticals simultaneously like a vascular agent and iodine in the blood, and you could then use a nanoparticle that targets cancer cells and goes to the tumor," Butler said. Teams are looking at "nanoparticles that metabolize in the body, so you put in the nanoparticle that contains both gold and iodine, and it will be metabolized within certain cells."
Of course, today's dual-energy CT scanners, and even single-source scanners with fast kV switching, can provide information about different targeted materials such as calcium and iodine. But many of these applications carry a cost in the form of higher radiation dose and/or reduced image quality, due in part to overlapping beam energies. Multienergy imaging on a dedicated scanner is expected to be faster and more efficient.
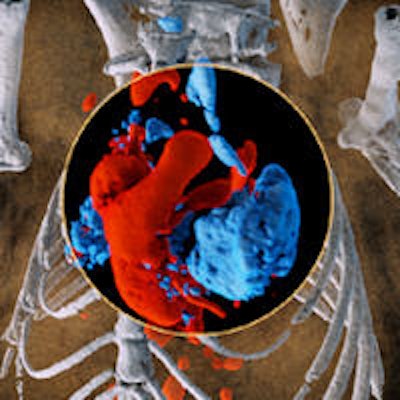
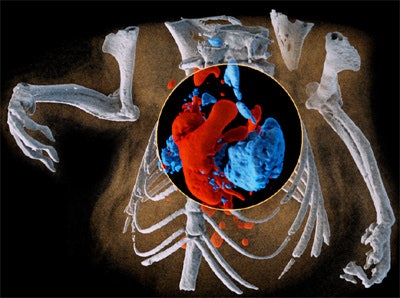 Multispectral imaging on a small scale: 3D image of mouse showing bone (white), iodine in the cardiovascular system (red), and barium in the lungs (blue). All images courtesy of Dr. Anthony Butler, PhD.
Multispectral imaging on a small scale: 3D image of mouse showing bone (white), iodine in the cardiovascular system (red), and barium in the lungs (blue). All images courtesy of Dr. Anthony Butler, PhD.New Zealand's global partnership
The half-dozen partners in the scanner project comprise several universities, including the University of Canterbury, which will build the scanner; Dunedin School of Medicine at the University of Otago, where the first scanner will be sited; and Lincoln University, which will provide large animals for testing the scanner, according to the University of Canterbury. GE Healthcare will provide some funding, along with the actual x-ray tube to be installed in the system.
The spectral technology they are using was first developed in the 1990s in partnership with the researchers who built Switzerland's CERN particle accelerator. Philips Healthcare also built a preclinical spectral scanner, and the University of Canterbury team consulted with the firm and with CERN and other imaging vendors in the development of its preclinical device a decade ago, he said.
The result of those efforts was the Medipix All Resolution System (MARS) spectral micro-CT scanner (commercialized by Mars Bioimaging in 2007). The system can now be found at research centers around the world, using the Medipix3 chip originally based on CERN technology to deliver the eight x-ray energies suitable for animal and human imaging.
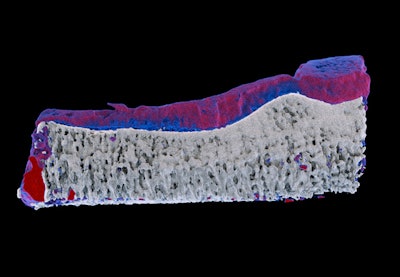 Measurements of biochemistry on the MARS preclinical spectral scanner enable early detection of arthritis. Red represents diseased cartilage; blue represents healthy cartilage. On traditional x-ray imaging, both areas are "gray," so arthritis can only be measured when the cartilage has been destroyed. The work was done in partnership with the University of Otago medical school's radiology and orthopedics departments.
Measurements of biochemistry on the MARS preclinical spectral scanner enable early detection of arthritis. Red represents diseased cartilage; blue represents healthy cartilage. On traditional x-ray imaging, both areas are "gray," so arthritis can only be measured when the cartilage has been destroyed. The work was done in partnership with the University of Otago medical school's radiology and orthopedics departments.Back in Christchurch, spirits have been high since the group learned earlier this month that the project funding had been approved. "We hope to have a full-scale system in about three years and put the first humans in in about five years," Butler said.
The project will be funded with 12 million New Zealand dollars, about $10 million U.S., Butler said. The universities will also provide faculty partners and as many as 30 doctoral students to work on the new project. Beyond GE's x-ray tube, most of the electronics are being manufactured in New Zealand.
Into action
To build the human scanner and apply the technology on a larger scale, the group will rely on the relationships it has built over the years with dozens of international universities and companies, including precision mechanical and electronics manufacturing firms.
New application development will focus on heart disease and bone implants such as hip replacements, as well as helping cancer researchers and drug developers try out new imaging compounds. The technology will help identify and direct treatments for unstable atherosclerotic plaque, assess activity and treatment response for a range of inflammatory diseases, and monitor cancer biomarkers and treatment, according to the University of Canterbury.
Five years ago, only a small number of people were working on this kind of research, but now many more researchers are involved and publishing their work on spectral imaging, Butler said.
"There's been a lot of work in Europe and in the states on these contrast agents," and pharmaceutical companies are developing agents specifically for use on the new scanner, he said. "There's quite a lot you can do with it."




















