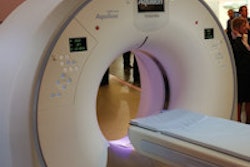
The U.S. Food and Drug Administration (FDA) has given Toshiba America Medical Systems clearance to sell its Infinix 4D CT interventional concept in a configuration outfitted with its Aquilion Prime CT scanner.
Infinix 4D CT combines an angiography system with a CT scanner in a single room to allow both technologies to be used during interventional procedures. Toshiba previously received clearance for Infinix 4D CT with the Infinix Elite cardiovascular x-ray system and Aquilion One Vision CT scanner.
Toshiba will display the new configuration at this week's American College of Cardiology (ACC) meeting in San Diego.