The use of the CT Colonography Reporting and Data System (C-RADS) has brought a standardized approach to risk assessment and management of findings both inside and outside the colon, paving the way to high-quality population-based colorectal cancer screening, according to an article in the Journal of the American College of Radiology.
Developed by CT colonography (CTC) experts a decade ago and based on the BI-RADS scheme for breast cancer evaluation, C-RADS defines lesion morphology, size, and number to provide an overall category of disease severity, wrote lead author Dr. Judy Yee from the University of California, San Francisco, along with colleagues Dr. Abraham Dachman, Dr. Kevin Chang, and others. Over a decade of clinical application, C-RADS has shown the utility of polyp size as the driver of patient management, the group wrote (JACR, June 1, 2016).
"By defining standard terms of lesion morphology along with lesion size and number, an overall category of disease severity may be assigned per patient," the authors wrote. "Beyond clarity of communication to referring physicians, this scheme also provides the foundation to better evaluate results in clinical research across different centers."
Finally, they added, "C-RADS can aid in the assessment of quality metrics in clinical use, which is used to drive reimbursement both in Medicare and in the private sector."
In the spotlight once again
CT colonography is once again in the spotlight with its June 15 approval as a colorectal cancer screening technique by the U.S. Preventive Services Task Force (USPSTF) -- just a step short of a favorable national coverage decision by the U.S. Centers for Medicare and Medicaid Services (CMS). This would enable Medicare reimbursement for population-wide screening; reimbursement is already widely available from private payors.
Developed by a working group that included members of the American College of Radiology's colon cancer committee and key opinion leaders in CTC, the C-RADS scheme was first published in Radiology in 2005 (Zalis et al) with the goal of emulating the success of BI-RADS in making CTC reporting more consistent and standardized.
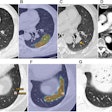
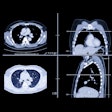
In C-RADS, lesions are described by size, morphology, and location in the colon. Polyps are soft-tissue attenuating structures described as sessile (broad-based), pedunculated (located on a stalk), or flat (less than 3 mm in height), the authors wrote.
Size is measured in millimeters as the single largest polyp diameter excluding the stalk. Finally, locations are described in terms of the six standard colonic segments, including the rectum, sigmoid colon, descending colon, transverse colon, ascending colon, and cecum, the team wrote.
Colorectal findings are categorized as C0 through C4:
- C0 indicates an incomplete study.
- C1 denotes a normal study.
- C2 signifies an exam with one or two 6- to 9-mm lesions.
- C3 denotes an exam with one or more polyps 10 mm or larger.
- C4 indicates suspected malignant masses.
Extracolonic findings are categorized as E0 through E4:
- E0 indicates a technically limited exam.
- E1 signifies a normal exam.
- E2 represents an exam with clinically unimportant findings.
- E3 is an exam with potentially important findings.
- E4 denotes an exam with potentially important findings that should be communicated to the referring physician.
Size defines lesion risk
"With respect to colonic findings, the categorization of polyps by size has demonstrated the utility of polyp size to drive patient management," Yee and colleagues wrote. Before C-RADS and CTC, the dominant practice was universal polypectomy, despite knowledge that the vast majority of polyps "would never transform to cancer and that size was a powerful biomarker to help stratify levels of risk," they wrote.
Large polyps are at risk for high-grade dysplasia, advanced histologic characteristics, and cancer or future risk thereof. Subcentimeter polyps, especially those 6 mm and smaller, "are unlikely to harbor advanced histology or cancer or to progress to cancer in the future in patients without family histories of colorectal cancer," the authors wrote.
C-RADS directs management
The use of C-RADS size categories in CT-based screening has enabled a major shift to size-based management, or selective polypectomy, in screening, according to the group. This approach has substantially reduced the number of benign or low-risk polyps being removed that would never have transformed to cancer, leaving those lesions with the highest risk to be removed, while those with smaller polyps 6 mm to 9 mm are typically placed into CTC surveillance of three years (and given the option of immediate colonoscopy). CTC follow-up in those with diminutive polyps can be delayed as long as five years.
A study examining parallel CTC and colonoscopic screening programs in more than 6,000 patients found that CTC led to a more than fourfold decrease in polypectomies (123 with CTC versus 121 with colonoscopy, p < 0.001) while maintaining the same number of resected advanced neoplasia (123 with CTC versus 121 with colonoscopy, p = 0.81), Yee and colleagues noted. Other reports have shown that surveillance of 6- to 9-mm polyps and nonreporting of isolated diminutive polyps are safe, with low rates of progression. The majority of small polyps remain stable or shrink over prescribed CTC surveillance intervals, the authors concluded.
Published screening studies have reported a combined rate of C-RADS E3 and E4 findings of 10% to 15%. Pooler et al examined almost 8,000 patients over eight years and found an E4 rate of only 2.5%.
"Nearly all of these 202 patients had confirmed relevant abnormalities after uncomplicated workup, demonstrating a strong net benefit" for detecting extracolonic findings, Yee and colleagues wrote.
Extracolonic tumors and abdominal aortic aneurysms are the most prevalent -- in fact, the frequency of these two findings may exceed the detection rate of colorectal cancer in a screening population, they wrote of the study. Hassan et al showed substantial gains in life years using a CTC screening strategy versus colorectal cancer screening with colonoscopy.
Few extracolonic findings
The rate of extracolonic findings can be expected to vary depending on the study cohort, but taken together, several studies that used C-RADS showed a limited range of 8.3% to 13.4%, the authors wrote. Badiani et al logged extracolonic findings in 23% of screening subjects, but workup was less than 5%. Macari et al found more extracolonic findings in seniors, but additional evaluation rate was limited to 6%, versus 4.4% for nonseniors.
Costs of following up findings in the C-RADS studies have been remarkably consistent at $28 to $50 per person, with a mean of less than $34. The largest C-RADS study looked at 7,679 asymptomatic adults undergoing CTC screening. In all, 0.7% of screening subjects were classified as C0 under C-RADS, 85% were classified as C1, 8.6% were C2, 5.2% were C3, and 0.6% were C4.
The C-RADS studies also showed that patients who were older and male had more positive findings. Moore et al reported slightly lower rates of positive findings in a cohort of 2,152 patients, with 4.6% for C2, 3.6% for C3, and 2.5% for C4 findings.
"Standardization of risk evaluation and follow-up is a prerequisite for the implementation of high-quality screening that is scalable to large populations," the study authors noted. "C-RADS represents a balance between a complete, nuanced description of relevant findings and a practical ready reference to promote standardized reporting."
As further evidence accrues, C-RADS and its recommended evaluation intervals may evolve, but the experience to date has demonstrated utility and broad acceptance of the reporting system, they concluded.