A new reconstruction algorithm can erase beam-hardening artifacts in myocardial perfusion imaging (MPI) in dual-source CT, bolstering the reliability of the increasingly popular examination, according to a U.S.-German study in European Radiology.
Researchers examined 14 patients with dual-source CT myocardial perfusion, creating six image series with several exposure settings for each patient. Each series was reconstructed both with and without the use of the beam-hardening correction kernel (D33F, Siemens Healthineers).
Among the more than 750 myocardial regions assessed, they found the regions prone to beam hardening were readable and diagnostic with use of the beam-hardening correction kernel versus images processed using a standard kernel (D30f).
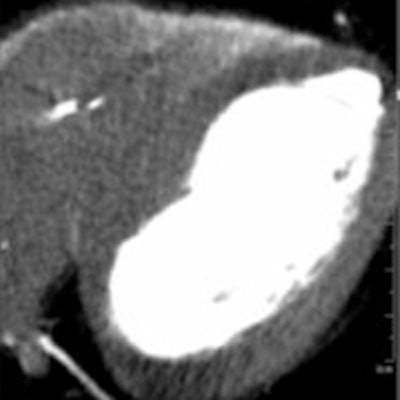
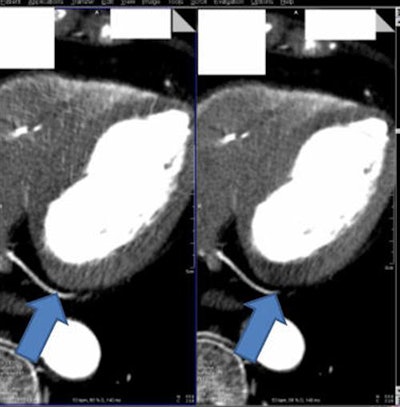 Myocardial CT image (above right) reconstructed without beam hardening correction kernel. Distinct beam-hardening artifact, mimicking hypodensity, such as found in myocardial infarct, is visible. The inhomogeneous dorsal wall of the left ventricle represents a possible myocardial infarct. The patient cannot be discharged and undergoes further (possibly) invasive examinations. Image above left shows same anatomy with beam-hardening correction algorithm applied. Homogeneous dorsal wall of the left ventricle confirms no myocardial infarct. With a high certainty in diagnosis, the patient can be discharged. Images below and bottom depict iodine maps of the same anatomy with no beam-hardening correction applied. All images courtesy of Drs. Albrecht Moritz and U. Joseph Schoepf.
Myocardial CT image (above right) reconstructed without beam hardening correction kernel. Distinct beam-hardening artifact, mimicking hypodensity, such as found in myocardial infarct, is visible. The inhomogeneous dorsal wall of the left ventricle represents a possible myocardial infarct. The patient cannot be discharged and undergoes further (possibly) invasive examinations. Image above left shows same anatomy with beam-hardening correction algorithm applied. Homogeneous dorsal wall of the left ventricle confirms no myocardial infarct. With a high certainty in diagnosis, the patient can be discharged. Images below and bottom depict iodine maps of the same anatomy with no beam-hardening correction applied. All images courtesy of Drs. Albrecht Moritz and U. Joseph Schoepf.When the algorithm was employed, the group found significantly lower overall mean myocardial attenuation than without it. Relative differences from average myocardial attenuation were also smaller with use of the algorithm (p < 0.01) (European Radiology, September 2016, Vol. 26:9, pp. 3,215-3,222.)
"We found that the dual-source CT-based algorithm does eliminate those areas of artifactual attenuation within the heart muscle, and restores a more realistic visualization of what it should look like without causing false-positives," lead investigator Dr. U. Joseph Schoepf from the Medical University of South Carolina told AuntMinnieEurope.com.
Universal problem
Beam-hardening occurs when a polychromatic x-ray beam passes through a material of higher atomic number such as bone or iodine due to the preferential absorption of lower- energy-spectrum radiation, the authors explained. The discrepancy between the actual attenuation and an assumed linear attenuation coefficient creates beam-hardening correction, which is seen as cupping artifacts and dark streaks in high-attenuation areas of the CT image.
All vendors are struggling with this issue, according to Schoepf.
"Often you have areas in the heart muscle that appear to have a perfusion defect, whereas in reality it's just caused by beam-hardening artifact. The beam gets artificially hardened by penetrating very hard structures such as the spine or the contrast-enhanced thoracic aorta," he said. "So when the beam travels through those very hard structures you alter the spectrum of your x-ray radiation, and that will cause some areas of the heart muscle to look hypoattenuated as if there were a perfusion defect."
Previous beam-hardening correction techniques have included hardware corrections built into the CT scanner, including filtration through thin metal plates that physically harden the x-ray beam spectrum and bowtie filters. Other methods are based on dual-energy CT acquisition such as blending datasets from both energy spectra or reconstructing virtual monochromatic images. Finally, postprocessing methods include statistical polychromatic reconstruction using maximum likelihood algorithms, and linearization, which generates a monochromatic attenuation profile.
Integrated beam-hardening correction methods such as the one proposed in this study can be helpful to workflow because they don't require additional postprocessing. The authors aimed to quantify the impact of an integrated beam-hardening correction algorithm on beam-hardening artifacts in the myocardium generated in dual-energy CT myocardial perfusion studies.
The study retrospectively analyzed rest-series dual-energy CT MPI exams from 14 patients (mean 62.9 years, six women). The investigators generated six separate image series for each patient, including 100 kV and 140 kV images, and linearly blended MIX0.5 images each using a beam hardening correction algorithm (D33f kernel) or a standard kernel (D30f kernel).
They were applied to regions of the myocardium prone to beam-hardening artifacts. Existing SPECT studies were used to confirm the presence and location of true perfusion defects, the authors wrote. Statistical evaluation was performed using paired student t-tests.
Artifacts erased with algorithm
A total of 756 myocardial regions were analyzed; six confirmed perfusion defects were located within the regions of interest.
An analysis of pooled data showed that overall mean myocardial attenuation was lower using the beam-hardening correction filter: 87.3 ± 24.1 HU versus 85.5 ± 21.5 HU without it (p = 0.009).
Second, the overall relative difference from average myocardial attenuation (RDMA) was more homogeneous using beam-hardening correction: -0.3 ± 11.4 % without the filter; 0.1 ± 10.1 using the novel D33f kernel (p < 0.001), Schoepf and colleagues reported.
Finally, the relative differences from average myocardial attenuation were greatest in the posterobasal myocardium: -16.2 ± 10.0% without the BHC kernel; 3.4 ± 10.7% with it (p < 0.001).
Using beam-hardening correction, the mean attenuation within identified areas of artifacts remained within one standard deviation of the mean Hounsfield unit levels for all three tube voltage levels, suggesting adequate correction in regions that would otherwise interfere with assessment, the group wrote.
"Hypoattenuation in regions with beam-hardening artifact was consistently corrected towards reference mean myocardial attenuation with the D33f kernel, providing a more homogeneous attenuation of the myocardium," the authors wrote. "The implementation of this beam-hardening correction kernel may thus improve assessment of CT MPI datasets in patients."
As for limitations, the small sample size precluded the ability to analyze for selectivity of perfusion defects between areas containing real perfusion defects and areas affected by beam-hardening artifacts. For this reason, all included patients had undergone SPECT within 30 days of CT myocardial perfusion to exclude potential bias due to the presence of actual perfusion defects.
Integrated beam hardening correction using the D33f kernel is feasible and allows for significant reduction of myocardial beam-hardening artifacts to improve attenuation of regularly perfused myocardium, the authors concluded. The results are in line with previous techniques, and offer the advantage of eliminating additional postprocessing.
"I don't believe you need a whole lot of patients to demonstrate the technical success of an application," Schoepf said. "It gets rid of most of the artifacts so it did a very good job in making the situation better," Schoepf said.
Typically such innovations don't require regulatory clearance, Schoepf added; they are simply incorporated into the next iteration of the vendor's reconstruction software.
The new algorithm can also be applied to single-energy CT, so its performance should also be evaluated in single-energy cardiac CT, particularly employing the commonly used 120 kV datasets, the authors wrote.