Royal Philips, the parent company of Philips Healthcare, has entered into an agreement with cardiovascular software developer HeartFlow for that company's fractional flow reserve (FFR) technology for evaluating and treating patients with suspected coronary artery disease.
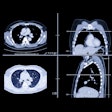
Philips will promote HeartFlow Analysis FFR-CT software in conjunction with its own advanced catheters for imaging and assessing measurements of the inside of a patient's coronary arteries. The advanced catheters include Philips' coronary and intravascular ultrasound devices.
The collaboration will initially focus on the U.S. with the intention to expand globally soon. The two companies have also entered into an exclusive agreement to co-develop an improved cath lab x-ray image-derived FFR or instant wave-free ratio (iFR) product to aid workflow and improve diagnosis and treatment.
In other Philips news, a new clinical trial presented at the European Society of Cardiology congress (ESC 2017) found that angiography alone may not be enough for determining treatment for complex coronary procedures.
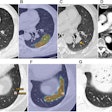
Dr. Javier Escaned of Hospital Clínico San Carlos in Madrid presented data from the SYNTAX II trial indicating that the percutaneous coronary intervention (PCI) technique might simplify procedures and contribute to improved patient outcomes. The PCI procedure incorporates physiology, intravascular ultrasound imaging, synergy drug-alluding stents, and medical therapy.
The trial included 454 patients from 22 centers across the U.K., the Netherlands, Spain, and Poland.