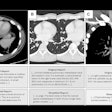
Canon Medical Systems USA has received U.S. Food and Drug Administration (FDA) 510(k) clearance for its artificial intelligence (AI)-based Advanced Intelligent Clear-IQ Engine (AiCE) image reconstruction technology.
First introduced at RSNA 2018, AiCE employs a deep convolutional neural network to suppress noise and enhance signal, according to the firm. It can reconstruct CT images with better spatial resolution than the company's adaptive iterative dose reduction (AIDR) 3D iterative reconstruction protocol and at a rate three to five times faster than traditional model-based iterative reconstruction (MBIR) methods, Canon said.