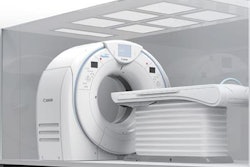
Canon Medical Systems USA has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for its Aquilion One / Prism edition CT scanner.
The scanner uses deep-learning spectral capabilities to allow for more routine spectral imaging. Through its artificial intelligence technology, the Aquilion One / Prism edition also is designed to enhance workflow and provide more detailed images and data to help physicians make more informed decisions about a patient's care.
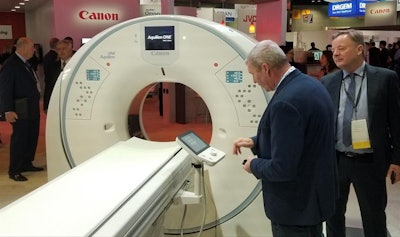 Canon's Aquilion One / Prism CT edition CT scanner.
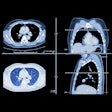
Canon's Aquilion One / Prism CT edition CT scanner.The scanner's advanced intelligent Clear-IQ Engine (AiCE) also uses deep learning to distinguish true signal from noise for enhanced image quality across the entire body, including brain, lung, cardiac, and musculoskeletal applications.