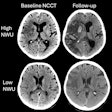
Artificial intelligence (AI) software developer Qure.ai has received U.S. Food and Drug Administration (FDA) 510(k) clearance for its qER AI triage software for emergency room (ER) head CT exams.
The software has been cleared for use in triaging CT scans containing intracranial bleeds, mass effect, midline shift, and cranial fractures, according to the vendor. As a result, qER can be used to triage nearly all critical abnormalities visible on routine head CT scans, the company said.