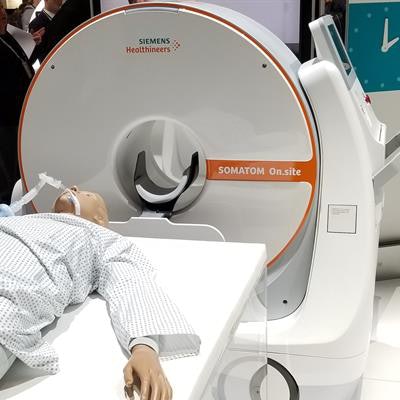
The U.S. Food and Drug Administration (FDA) has granted 510(k) clearance for the Somatom On.site portable head CT scanner from Siemens Healthineers.
First demonstrated at the RSNA 2019 meeting, Somatom On.site is a portable CT scanner on a portable motorized trolley that can be moved to a patient's bedside. The 32-slice scanner has a 0.7-mm detector width, providing 2.4 cm of coverage per gantry rotation.
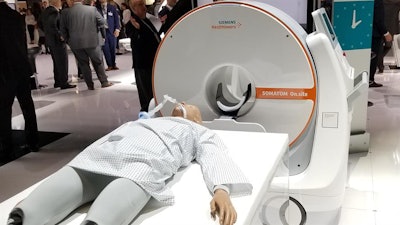 The Somatom On.site portable head CT scanner from Siemens Healthineers. Image courtesy of Siemens Healthineers.
The Somatom On.site portable head CT scanner from Siemens Healthineers. Image courtesy of Siemens Healthineers.On.site has an integrated drive camera that enables real-time viewing on the built-in touch display, and the scanner's telescopic gantry design allows the radiation source to move away from the patient during scanning, while the base and the front cover of the gantry remain stationary. Images acquired with On.site can be sent to PACS wirelessly.



















