Deep learning-based analysis of CT biomarkers can enable the diagnosis of type 2 diabetes mellitus on noncontrast abdominal CT exams performed to screen for CT colonography (CTC), according to research published online April 5 in Radiology.
Researchers from the University of Wisconsin School of Medicine and Public Health in Madison, WI, and the U.S. National Institutes of Health (NIH) Clinical Center in Bethesda, MD, developed an automated pancreatic segmentation method for noncontrast CT images. They then tested the algorithm retrospectively on 9,000 consecutive patients who had undergone CTC screening.
They found that the five most important CT biomarkers extracted using the deep learning-based segmentation model yielded an area under the curve (AUC) of 0.81 on a subset of patients with CT exams performed zero to 2,550 days before their diagnosis of type 2 diabetes.
"This study is a step toward the wider use of automated methods to address clinical challenges," wrote the researchers led by first author Hima Tallam, a medical and doctoral student at the University of Wisconsin. Dr. Perry Pickhardt of the University of Wisconsin and Dr. Ronald Summers, PhD, of the NIH Clinical Center served as co-senior authors.
A number of research studies have explored the utility of utilizing information on CT images to opportunistically perform screening for other conditions beyond the clinical indication of the study, including osteoporosis, cardiovascular events, and metabolic syndrome. In the current study, the researchers sought to investigate the utility of an automated deep-learning approach for detecting type 2 diabetes via CT biomarkers.
The researchers used data from 471 images -- including both contrast-enhanced CT and noncontrast CT images -- from a variety of public datasets to train a deep-learning algorithm to segment the pancreas. The study participants had a diabetes diagnosis that ranged from 5,055 days before diabetes diagnosis to 4,822 days after diagnosis.
In testing on a subset of 25 cases, the deep learning-based pancreas segmentations were deemed to be accurate and reproducible, with a Dice similar coefficient of 0.69 that was equivalent to the interobserver performance for two human readers.
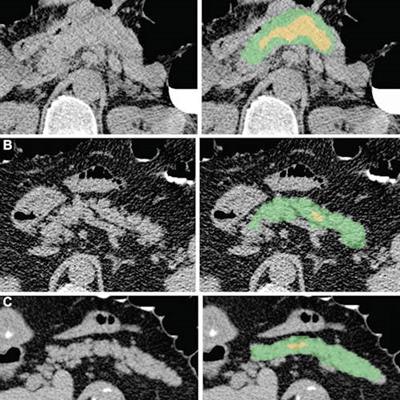
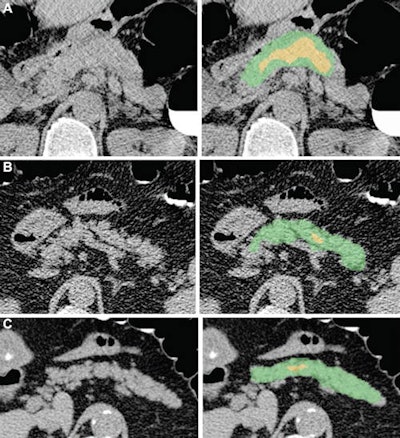 Examples of pancreas segmentations on unenhanced axial abdominal CT images in healthy participants and patients with type 2 diabetes mellitus. Images on left are original CT images, and images on right show segmentations overlaid on the original CT images. (A) Images in a nondiabetic 61-year-old man with average pancreas CT attenuation of 35.50 HU ± 47.96 and pancreatic volume of 97.6 mL. (B) Images of a 59-year-old man with type 2 diabetes who was diagnosed 144 days before CT. Average pancreas CT attenuation was 20.66 HU ± 81.99 and pancreatic volume was 77.10 mL. (C) Images in a 67-year-old man with type 2 diabetes who was diagnosed 595 days after CT. Average pancreas CT attenuation was 18.46 HU ± 48.30 and pancreatic volume was 72.88 mL. The green area indicates full segmentation and the yellow area indicates segmentation after erosion. Image and caption courtesy of the RSNA.
Examples of pancreas segmentations on unenhanced axial abdominal CT images in healthy participants and patients with type 2 diabetes mellitus. Images on left are original CT images, and images on right show segmentations overlaid on the original CT images. (A) Images in a nondiabetic 61-year-old man with average pancreas CT attenuation of 35.50 HU ± 47.96 and pancreatic volume of 97.6 mL. (B) Images of a 59-year-old man with type 2 diabetes who was diagnosed 144 days before CT. Average pancreas CT attenuation was 20.66 HU ± 81.99 and pancreatic volume was 77.10 mL. (C) Images in a 67-year-old man with type 2 diabetes who was diagnosed 595 days after CT. Average pancreas CT attenuation was 18.46 HU ± 48.30 and pancreatic volume was 72.88 mL. The green area indicates full segmentation and the yellow area indicates segmentation after erosion. Image and caption courtesy of the RSNA.Logistic regression analysis then showed that, on average, patients with type 2 diabetes had lower average pancreas, muscle, and liver CT attenuation; higher standard deviation in pancreas CT attenuation; and lower pancreas fractal dimension. A progressive decrease in pancreatic attenuation was found in patients with a greater disease duration.
"These findings point to high amounts of peri-organ and intra-organ fat, which are related to and consistent with previous work showing that patients with diabetes tend to accumulate more visceral and intrapancreatic fat than do persons without diabetes," the authors wrote.
Utilizing these CT biomarkers, the deep-learning model was able to achieve a high level of accuracy for diagnosing type 2 diabetes.
| Performance of deep learning-based CT segmentation model for diagnosing type 2 diabetes | |
| Detecting diabetes from 0-2,550 days before diabetes diagnosis | AUC = 0.81 |
| Detecting diabetes from 0-2,550 days after diabetes diagnosis | AUC = 0.84 |
Adding clinical information into the model did not significantly improve performance.
The researchers said that future work may focus on predicting type 2 diabetes in a prospective study. In addition, the current study may also inform future research on the reasons why morphologic characteristics of the pancreas change in patients with diabetes, they said.
"However, we ultimately hope that the CT biomarkers investigated herein might inform diagnosis of early stages of type 2 diabetes and allow patients to make lifestyle changes to alter the course," the authors wrote.