Artificial intelligence (AI) software developer Annalise.ai has secured clearances from the U.S. Food and Drug Administration (FDA) that cover additional indications for use of its CT and x-ray triage and notification software.
Annalise Triage uses deep learning to identify the suspected presence of time-sensitive pathologies and flag priority cases within the radiology worklist, assisting the care team in treating patients with the most critical conditions first.
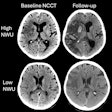
New clearances for the company's Triage CT Brain algorithm include the following:
- Acute subdural/epidural hematoma
- Acute subarachnoid hemorrhage
- Intra-axial hemorrhage
- Intraventricular hemorrhage
In addition, clearances for the company's Triage Chest X-ray algorithm now include the following:
- Pneumothorax
- Tension pneumothorax
- Pleural effusion
- Pneumoperitoneum
- Vertebral compression fracture
The latest FDA clearances will allow the company to expand into the U.S. market and bring the total number of U.S.-available findings for its triage and notification products to nine, Annalise.ai representatives said.