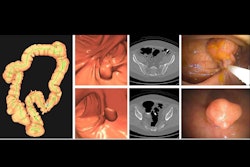
At long last, the U.S. Centers for Medicare and Medicaid Services (CMS) is now backing Medicare reimbursement for CT colonography (CTC) for colorectal cancer screening.
The CMS has included coverage of CTC in its proposed 2025 Hospital Outpatient Prospective Payment System (HOPPS) and 2025 Medicare Physician Fee Schedule (MPFS) proposed rules, which were published on July 10. The American College of Radiology (ACR), which has been lobbying the CMS to provide Medicare coverage for CTC for over 15 years, lauded the move, marking it as "a big step forward toward providing Medicare patients access to a minimally invasive colorectal cancer screening tool that can detect precancerous polyps and does not require anesthesia."
Colorectal cancer is the leading cause of cancer death in men under 50 in the U.S., and the burden of disease is higher for Black Americans, who are more than 20% more likely to get colorectal cancer and 40% more likely to die of it compared with their white or Hispanic peers, the ACR noted. The organization's lobbying efforts began after it published its American College of Radiology Imaging Network National CT Colonography Trial in 2008; the American Cancer Society, the U.S. Preventive Services Task Force, and the U.S. Food and Drug Administration also support coverage, but the CMS denied it in 2009 and had continued to do so until now.
After considering recommendations as well as guidelines from appropriate organizations, the CMS wrote that it believes “CTC to be reasonable and necessary as CRC screening test, especially for patients and clinicians who seek a direct visualization procedure as a first step in CRC screening that is less invasive and less burdensome on the patient and healthcare system compared with Screening Colonoscopy. Our goal is that the patient and their clinician make the most appropriate choice in CRC screening, which includes considerations of the risks, burdens, and tradeoffs for each covered test or procedure. We expect that clinicians who order CTC for CRC Screening will educate their patients on risks and context of radiation exposure and potential extracolonic findings.”
Coverage of CTC for colorectal cancer screening (CRC) would replace barium enema screening.
The CMS proposed that CTC coverage be limited to the following:
- In the case of an individual age 45 or over who is not at high risk of colorectal cancer, payment may be made for a screening CT colonography performed after at least 59 months have passed following the month in which the last screening CTC exam or 47 months have passed following the month in which the last screening flexible sigmoidoscopy or screening colonoscopy was performed.
- In the case of an individual who is at high risk for colorectal cancer, payment may be made for a screening CTC exam performed after at least 23 months have passed following the month in which the last screening CTC or the last screening colonoscopy was performed.
“We recognize there are several advantages to choosing a noninvasive CRC screening test as a first step compared to a screening colonoscopy, including relative ease of administering the test and potentially reducing the experience of burdensome preparation and invasive procedures,” the CMS wrote in the proposed rule.
Also, the CMS expanded its approach to “complete CRC screening” to include a covered blood-based biomarker test alongside a covered noninvasive stool-based test.