Epicardial adipose tissue (EAT) on low-dose CT (LDCT) images is tied to higher cardiovascular mortality risk in lung cancer patients, according to research published February 18 in Radiology.
A team led by Isabel Langenbach, MD, from Massachusetts General Hospital and Harvard Medical School in Boston found that EAT volume increase and decrease and EAT density increase beyond typical on subsequent chest CT scans were linked to all-cause mortality in participants screened for lung cancer.
“EAT volume decrease and EAT density increase were associated with elevated risk of cardiovascular mortality after adjustment for baseline EAT values,” the Langenbach team wrote.
Along with being the main cause for developing lung cancer, smoking is also tied to cardiovascular disease development. Previous reports suggest that cardiovascular disease risk is higher than lung cancer risk in smokers.
And while low-dose CT is the go-to modality for lung cancer screening, it also captures images of the heart. The researchers highlighted that this may be an area for health professionals to capitalize on.
Epicardial adipose tissue is a fatty deposit found between the myocardium and the visceral layer of the pericardium that surrounds the coronary arteries and the heart. Previous research suggests it is related to atherogenesis and cardiovascular disease. And low-dose CT can be used to evaluate EAT.
Langenbach and colleagues studied how longitudinal changes in EAT relate to mortality in individuals undergoing serial low-dose CT lung cancer screening as part of the National Lung Screening Trial (NLST).
The secondary analysis of the NLST included data from 20,661 participants, including EAT volume and density from serial low-dose CT scans that used a validated automated deep learning algorithm.
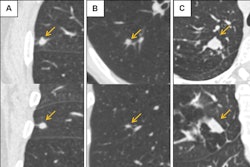
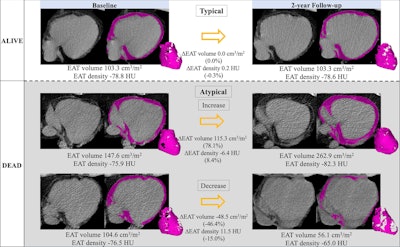 Epicardial adipose tissue (EAT) segmentation (shading) at noncontrast chest CT in axial orientation. Representative examples show EAT changes (ΔEAT) between baseline and two-year follow-up CT scans in participants who survived (typical changes) and increasing and decreasing EAT in participants who died. Images and caption courtesy of the RSNA.
Epicardial adipose tissue (EAT) segmentation (shading) at noncontrast chest CT in axial orientation. Representative examples show EAT changes (ΔEAT) between baseline and two-year follow-up CT scans in participants who survived (typical changes) and increasing and decreasing EAT in participants who died. Images and caption courtesy of the RSNA.
The researchers categorized EAT volume and density over two years into the following: typical changes, a decrease of 7% to an increase of 11% and decrease of 3% to increase of 2%, respectively; and atypical changes, increases, or decreases beyond typical ones.
Among the total participants, 3,483 (16.9%) died over a median follow-up of 10.4 years. Of these deaths, 816 (23.4%) were cardiovascular-related and 705 (20.2%) were lung cancer-related.
The researchers reported the following findings in relation to EAT changes:
- Average epicardial adipose tissue volume increased (2.5 cm3/m2) and density decreased (decrease of 0.5 Hounsfield units) over two years.
- Atypical changes in EAT volume were independent predictors of all-cause mortality. For atypical increase, the hazard ratio (HR) was 1.15 (p < 0.001); for atypical decrease, the HR was 1.34 (p < 0.001).
- An atypical decrease in epicardial adipose tissue volume was significantly tied to cardiovascular mortality (HR, 1.27; p = 0.009).
- EAT density increase was significantly linked to the following mortality types: all-cause (HR, 1.29; p < 0.001), cardiovascular (HR, 1.29; p = 0.005), and lung cancer (HR, 1.3; p = 0.007).
The study authors suggested that assessing epicardial adipose tissue changes over time could improve risk stratification in patients being screened for lung cancer, along with improving cardiovascular risk management.
In an accompanying editorial, Cristina Fuss, MD, PhD, from Yale School of Medicine in New Haven, CT, agreed that EAT assessments could improve early detection of pulmonary and cardiovascular risks.
“Such dual-purpose screening examinations could lead to more personalized and effective interventions that address the full spectrum of health threats faced by individuals with an extensive smoking history,” Fuss wrote.
She added that the study also “sets the stage” for future research to further uncover how EAT dynamics influence cardiovascular health.
“Prospective studies and randomized controlled trials are essential to confirm these findings and explore the potential of EAT changes as markers for therapeutic efficacy in CVD management,” Fuss wrote.
The full study can be found here.