This Princeton Junction, NJ, company will tout a recent alliance with multimodality vendor Philips Medical Systems, as well as a new enterprise-based version of its computer-aided detection (CAD) software for chest radiography studies.
EDDA inked the deal with Philips of Andover, MA, in April 2006. Philips gains exclusive worldwide rights to IQQA-Chest, which is designed to analyze digital radiography (DR) chest images and highlight suspicious lesions for radiologists. The software, which received U.S. Food and Drug Administration (FDA) clearance in 2004, will be made available with the company's DR systems.
Also at the RSNA meeting, University of Iowa researchers who have been working with the software since January 2006 will report on their experiences. Their study suggests that the software could help residents detect approximately 20% more small lung nodules, according to EDDA.
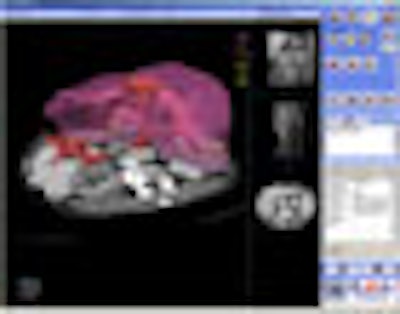
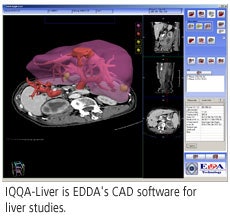
EDDA will also highlight its IQQA-Liver application, which will be shown as a work-in-progress. IQQA-Liver is designed to analyze serial multiphasic CT liver studies for the detection of liver disease, and also includes segmentation and measurement tools for visualizing and analyzing liver lesions and vascular structures. The software has been cleared by Chinese regulatory authorities and is undergoing review by the FDA.
By Brian Casey
AuntMinnie.com staff writer
November 3, 2006



















