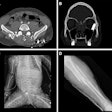
Carestream Health has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for its Carestream DRX-Evolution.
DRX-Evolution incorporates the company's DRX-1 cassette-sized wireless digital radiography (DR) detector in a fully automated suite. DRX-Evolution makes it possible for healthcare facilities to upgrade to a single- or dual-detector DR suite.
The Rochester, NY-based company plans to take orders immediately, with shipments beginning this fall.
Related Reading
Carestream adds DR order, July 29, 2009
Carestream scores Dutch contract, July 28, 2009
Carestream taps new India director, July 27, 2009
Carestream nets digital x-ray orders, July 22, 2009
Carestream sells RIS/PACS to Tufts, July 16, 2009
Copyright © 2009 AuntMinnie.com