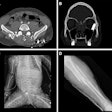
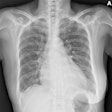
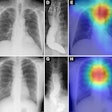
Digital radiography (DR) product developer ImaSight has received clearance from the U.S. Food and Drug Administration (FDA) for its ImaSight 4600 DR sensor.
ImaSight 4600 is based on CCD technology with an imaging surface of 16 million pixels, which the Gatineau, Quebec-based company claims addresses shortcomings in detectors with smaller or low-resolution CCDs.
The FDA issued the clearance on August 25.
Related Reading
ImaSight unveils DR sensor, January 23, 2007
Copyright © 2009 AuntMinnie.com