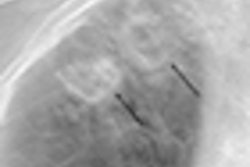
X-ray technology developer InfiMed of Liverpool, NY, has received clearance from the U.S. Food and Drug Administration (FDA) for its i5DR and i5 RF/DSA digital radiography (DR) systems, the company said.
i5DR and i5 RF/DSA are both part of InfiMed's new iSeries product line. Each system features InfiVision image processing technology that helps use the lowest possible dose for the highest image quality, according to InfiMed.
i5DR features an upgraded database for improved system throughput and enhanced GUI navigation for ease of use, and can integrate with various manufacturers' static flat-panel digital detectors and generators. The i5 RF/DSA system features new software updates that make acquiring images faster and easier, InfiMed said.
Related Reading
InfiMed, Carestream to integrate DR, October 16, 2009
InfiMed releases i4 DR, names Kogler as VP R&D, June 9, 2009
InfiMed ships first iSeries package, April 23, 2009
InfiMed to show DR/RF system at ECR, February 18, 2009
Road to RSNA, Digital X-Ray, InfiMed, October 22, 2008
Copyright © 2010 AuntMinnie.com