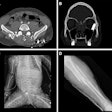
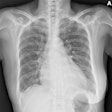
Digital radiography (DR) manufacturer DRGEM has received U.S. Food and Drug Administration (FDA) 510(k) clearance for its Diamond universal DR system.
Diamond employs a 17 x 17-inch amorphous silicon flat-panel detector from Varian Medical Systems of Palo Alto, CA, along with DRGEM's high-frequency generator, according to the Seoul, Korea-based firm. It also features automatic patient positioning capability, DRGEM said.
U.S. sales for Diamond will be handled by DRGEM USA of Gainesville, FL.
Copyright © 2011 AuntMinnie.com