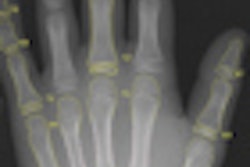
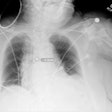
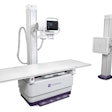
PACS integration company Meridian Medical Systems has received U.S. Food and Drug Administration (FDA) 510(k) clearance for its Universal Digital Interface (UDI) 1717 flat-panel digital upgrade.
Designed to compliment analog radiographic systems, UDI 1717 includes a 43 x 43-cm cesium iodide detector and an acquisition console. It can be configured with one or two digital panels.