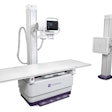
X-ray technology developer InfiMed has received U.S. Food and Drug Administration (FDA) 510(k) clearance for its i5 DR digital imaging software integrated with Samsung's SDX-4336CP portable flat-panel digital radiography (DR) detector.
InfiMed said that i5 DR is suitable for installation into any new or existing room or mobile x-ray system.