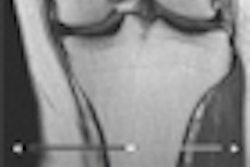
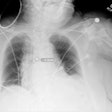
Medical imaging software developer MIM Software has become the first vendor to receive U.S. Food and Drug Administration (FDA) clearance for a mobile app for viewing diagnostic x-rays.
The company received 510(k) clearance for version 3.0 of its Mobile MIM application, which is now cleared for diagnostic use with x-ray and ultrasound, as well as for review and approval of radiation treatment plans. Mobile MIM received its initial 510(k) clearance earlier this year for diagnostic use on Apple's iPhone and iPad to view CT, MR, and nuclear medicine images (including PET).
""For images that require timely review, x-rays are the workhorse," MIM Software software director Jerimy Brockway told AuntMinnie.com. "Today, Mobile MIM brings these x-rays into the hands of diagnosticians."
Mobile MIM 3.0 can display images of more than 25 megapixels with lossless data compression, according to the firm. The company also noted that radiation oncologists can now use the app to review dose volume histograms, isodose curves, contours, and images for treatment plans, activities that have typically been restricted to a dedicated workstation.
Mobile MIM 3.0 is now available in the Apple App Store. It requires iOS 5 on either the iPad or iPhone, Brockway said.
MIM Software said that it also plans to launch a cobranded version of Mobile MIM with partner radiation therapy firm Accuray. Called PlanTouch, the version will have an interface that allows physicians to review and approve a CyberKnife treatment plan via a direct link, according to the vendor.