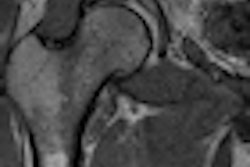
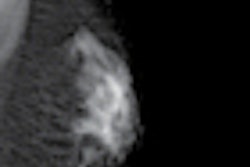
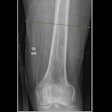
Crux Biomedical has received U.S. Food and Drug Administration (FDA) clearance for its inferior vena cava filter (VCF) with bidirectional retrieval.
The Crux VCF is designed to facilitate bidirectional retrieval through either the femoral or jugular veins, according to the company. In a recently completed trial presented at the 2012 Society for Interventional Radiology (SIR) meeting, researchers found a 98% success rate for both filter deployment and retrieval in 125 patients from 22 sites in the U.S., Australia, New Zealand, and Belgium.
No embolizations, migrations, or fractures were observed at six-month follow-up, Crux said.