Siemens Healthcare has received clearance from the U.S. Food and Drug Administration (FDA) for its Artis Q and Artis Q.zen angiography system product lines.
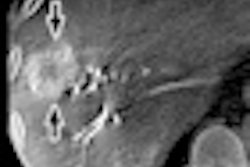
Both devices feature a new x-ray tube that helps physicians identify small vessels up to 70% better than conventional x-ray tube technology, according to Siemens. Artis Q.zen combines this x-ray source with a new detector technology that supports interventional imaging in ultralow-dose ranges.
Siemens will highlight the two new angiography product lines at the upcoming American College of Cardiology (ACC) meeting in San Francisco.
The company will also demonstrate release 3.0 of its Acuson SC2000 volume imaging ultrasound system at the ACC 2013 meeting. Designed to expand the usability of SC2000 into pediatric echocardiography applications, the new release includes a neonatal transducer that is optimized for pediatric imaging, with improvements in color Doppler and true fidelity color Doppler processing, Siemens said.