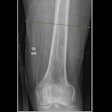
Women's imaging vendor Hologic has received U.S. Food and Drug Administration (FDA) approval for its single-energy femur exam, which detects atypical femur fractures on a dual-energy x-ray absorptiometry (DEXA) bone densitometer.
The system is part of a new line of bone densitometers the company will soon commercialize on its Horizon DXA platform, Hologic said. The exams are acquired in 15 seconds at very low radiation doses and produce high-resolution images of the entire femur, Hologic said.
Assessment for atypical femur fractures (AFF), a debilitating fracture associated with bisphosphonate therapy, can be conveniently performed at the time of a hip bone density scan with little or no patient repositioning and only a minimal increase in exam time, Hologic said. AFFs are uncommon, but they are much more debilitating than typical osteoporosis-related femur fractures.