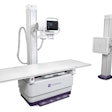
20/20 Imaging has received U.S. Food and Drug Administration (FDA) clearance for its HF PXS-710 digital radiography (DR) system.
The system is targeted for the podiatry market and offers the potential for lower radiation dose, according to the company. It can also be anatomically preprogrammed.