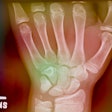
Portable x-ray system manufacturer Source-Ray has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for its UC-5000 digital radiography system.
The UC-5000 is designed for clinical and radiographic applications in the urgent care market. It features standard 115 AC voltage and requires only minimal shielding, according to the company.Source-Ray gets FDA OK for digital x-ray system
Latest in Digital X-Ray
Orthoscan debuts ultraportable mini C-arm at RSNA
November 30, 2025
Electrical bone stimulation may improve bone health in women
November 25, 2025
AI detects meningiomas on skull x-rays
November 21, 2025