Orthopedic imaging technology developer EOS Imaging has launched its EOSedge orthopedic digital radiography system in Europe and Canada and has received U.S. Food and Drug Administration 510(k) clearance for the system.
 EOSedge orthopedic digital radiography system. Image courtesy of EOS Imaging.
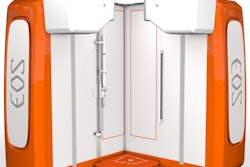
EOSedge orthopedic digital radiography system. Image courtesy of EOS Imaging.The next-generation system, which will also be showcased at next week's RSNA 2019 meeting in Chicago, features a new open-cabin design, the firm's Flex Dose technique, and high-resolution photon-counting detecting technology, according to the vendor.
The new open cabin includes a motorized, enlarged patient platform to accommodate a broader range of patients, EOS said. Flex Dose modulates radiation dose along the patient's body, while the high-resolution photon-counting detecting technology is designed to provide optimal image quality for musculoskeletal radiography, according to the vendor.
The EOSedge unit was installed earlier this year at the Imagerie Médicale du Créqui in Lyon, France. The company noted that it will also continue to offer its first-generation system.