
The U.S. Food and Drug Administration (FDA) has cleared the Multix Impact C ceiling-mounted digital radiography (DR) system from Siemens Healthineers. The FDA also cleared Multix Impact VA20, a new version of the company's existing floor-mounted DR system.
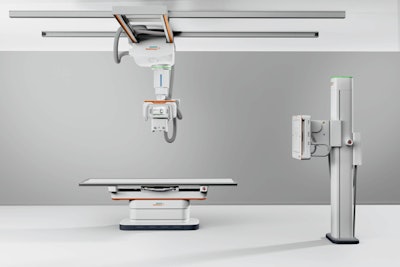 The Multix Impact C DR system. Image courtesy of Siemens.
The Multix Impact C DR system. Image courtesy of Siemens.Both Multix Impact C and Multix Impact VA20 employ a similar operating system, wireless digital detectors, motorized tube heads, and free-floating tabletop. Siemens is touting the touchscreen user interface on the x-ray tube head, which enables radiologic technologists to remain at the patient's side for longer periods. Patients can also be monitored with the system's myExam 2D camera.
Siemens introduced both systems at the RSNA 2020 meeting.



















