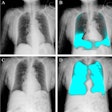
Artificial intelligence (AI) software developer Radiobotics has secured U.S. Food and Drug Administration 510(k) clearance for RBknee, an AI-based software application designed to aid in the diagnosis of knee osteoarthritis (OA) on radiography.
Suitable for use in orthopedic or radiological settings, RBknee analyzes digital x-rays of knees and identifies common radiographic findings associated with the diagnosis of OA: osteophytes, subchondral sclerosis, and joint space narrowing, according to the Copenhagen, Denmark-based vendor. Its machine-learning algorithms then conclude whether OA is present.
RBknee also measures the joint space width in both compartments of the knee, Radiobotics noted. Radiobotics received the CE Mark in Europe for RBknee earlier this year.